
- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-DYKDDDDK Tag magnetic beads
- Anti-DYKDDDDK Tag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Choose Your Country or Region
-
 Australia
Australia
-
 Austria
Austria
-
 Belgium
Belgium
-
 Brazil
Brazil
-
 Canada
Canada
-
 China
China
-
 Czech Republic
Czech Republic
-
 Denmark
Denmark
-
 Finland
Finland
-
 France
France
-
 Germany
Germany
-
 Greece
Greece
-
 Hong Kong
Hong Kong
-
 Hungary
Hungary
-
 Iceland
Iceland
-
 India
India
-
 Ireland
Ireland
-
 Israel
Israel
-
 Italy
Italy
-
 Japan
Japan
-
 Korea
Korea
-
 Luxembourg
Luxembourg
-
 Malaysia
Malaysia
-
 Netherlands
Netherlands
-
 New Zealand
New Zealand
-
 Norway
Norway
-
 Poland
Poland
-
 Qatar
Qatar
-
 Romania
Romania
-
 Saudi Arabia
Saudi Arabia
-
 Singapore
Singapore
-
 Spain
Spain
-
 Sweden
Sweden
-
 Switzerland
Switzerland
-
 Taiwan
Taiwan
-
 Turkey
Turkey
-
 United Kingdom
United Kingdom
-
 United States
United States
-

Home
Blog of Signal Transduction
PI3K/Akt/mTOR
PI3K
Endophilin plays a central role in endocytic pathway without calthrin
Category
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- CD markers
- PD-1/PD-L1
- TNF-alpha
- COX
- CCR
- Histamine Receptor
- IL Receptor
- MALT
- LTR
- TLR
- NOS
- Nrf2
- ROS
- NADPH-oxidase
- Anti-infection
- AhR
- gp120/CD4
- IDO/TDO
- CXCR
- NOD
- Immunology & Inflammation related
- Complement System
- Prostaglandin Receptor
- Glutathione
- SPHK
- β-lactamase
- PGES
- TBK1
- IFN
- IRAK
- FLAP
- Interleukins
- Arginase
- NLRP3
- Pyroptosis
- cGAS
- Neuraminidase
- SIK
- PGDS
- FKBP
- TRIF
- AKR1C
- PKR
- TpoR
- Parasite
- Heme Oxygenase
- MmpL3
- MyD88
- eIF
- Antioxidant
- STING
- MIF
- Galectin
- Nur77
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Stem Cells & Wnt
- Hippo
- Ubiquitin
- Neuronal Signaling
- Calcium Channel
- Beta Amyloid
- 5-HT Receptor
- COX
- GluR
- Adrenergic Receptor
- AChR
- Histamine Receptor
- Dopamine Receptor
- Opioid Receptor
- GABA Receptor
- P-gp
- P2 Receptor
- Cannabinoid Receptor
- OX Receptor
- CGRP Receptor
- MT Receptor
- MAO
- FAAH
- GlyT
- NMDAR
- CaMK
- BACE
- Trk receptor
- BChE
- EAAT
- Neurokinin Receptor
- Notch
- Imidazoline Receptor
- Sigma Receptor
- NPY receptor
- Serotonin Transporter
- CCK receptor
- COMT
- Neurotensin Receptor
- Melanocortin Receptor
- Adenosine Deaminase
- TRP Channel
- Adenosine Kinase
- NF-κB
- GPCR & G Protein
- Ras
- 5-HT Receptor
- CCR
- GluR
- Adrenergic Receptor
- AChR
- Histamine Receptor
- Dopamine Receptor
- Opioid Receptor
- GPR
- P2 Receptor
- Cannabinoid Receptor
- Endothelin Receptor
- S1P Receptor
- Hedgehog/Smoothened
- SGLT
- LPA Receptor
- OX Receptor
- CGRP Receptor
- MT Receptor
- PAFR
- PKA
- LTR
- Adenosine Receptor
- Vasopressin Receptor
- cAMP
- CXCR
- TAAR
- CaSR
- Glucagon Receptor
- PKG
- CRFR
- Angiotensin Receptor
- Bradykinin Receptor
- Imidazoline Receptor
- Bombesin Receptor
- RGS
- Neurotensin Receptor
- Sigma Receptor
- Melanocortin Receptor
- PAR
- Taste Receptor
- NPY receptor
- Parathyroid Hormone Receptor
- DOCK
- AdipoR
- GPCR19
- Guanylate Cyclase
- GHSR
- CCK receptor
- GRK
- Leukotriene
- FPR
- Prostaglandin Receptor
- PKD
- TSH Receptor
- Neurokinin Receptor
- GNRH Receptor
- Endocrinology & Hormones
- Transmembrane Transporters
- Metabolism
- PPAR
- P450 (e.g. CYP17)
- HSP (HSP90)
- PDE
- Hydroxylase
- Factor Xa
- DHFR
- Aminopeptidase
- Dehydrogenase
- Procollagen C Proteinase
- Phospholipase (e.g. PLA)
- DPP
- Carbonic Anhydrase
- Phosphorylase
- Liver X Receptor
- FAAH
- Acyltransferase
- FXR
- Transferase
- Serine/threonin kinase
- CETP
- IDO/TDO
- phosphatase
- GLUT
- HMG-CoA Reductase
- Vitamin
- PKM
- Lipoxygenase
- FOX
- Lipase
- Fatty Acid Synthase
- LDL
- NAMPT
- Decarboxylase
- Casein Kinase
- Thioredoxin
- Retinoid Receptor
- Mitochondrial Metabolism
- Peroxidases
- SHIP
- PCSK9
- PGDS
- Mitochondrial pyruvate carrier
- PREP
- Neprilysin
- PP2A
- FTO
- RUNX
- SREBP
- AdipoR
- PAD
- SCD
- CPT
- AP-1
- LDH
- Carbohydrate Metabolism
- CAR
- SSTR
- PAI-1
- γGCS
- SOD
- ATP-citrate lyase
- FAO
- FTase
- GOT
- 3-MST
- GTPCH
- SGK
- Serine hydrolase
- Epoxide Hydrolase
- glucocerebrosidase
- FABP
- Catalase
- THR
- ACSS2
- ROR
- Glucosylceramide Synthase
- ACE
- Thioredoxin Reductase
- PDHK
- PARG
- Xanthine Oxidase
- Mannosidase
- PGC-1α
- Adenosine Deaminase
- Aldose Reductase
- CPSase
- Leukotriene
- Adenosine Kinase
- OXPHOS
- Glutathione
- Acetyl-CoA carboxylase
- Proteases
- Microbiology
- Others
- ADC Cytotoxin
- ADC Linker
- Drug-Linker Conjugates for ADC
- Antineoplastic and Immunosuppressive Antibiotics
- Selection Antibiotics for Transfected Cell
- Antibiotics for Mammalian Cell Culture
- Antibiotics for Plant Cell Culture
- Hydrotropic Agents
- Dyes
- PROTAC
- PROTAC Linker
- E3 ligase Ligand
- Target Protein Ligand
- Others
- Antibody-Drug Conjugate
Archives
Endophilin plays a central role in endocytic pathway without calthrin
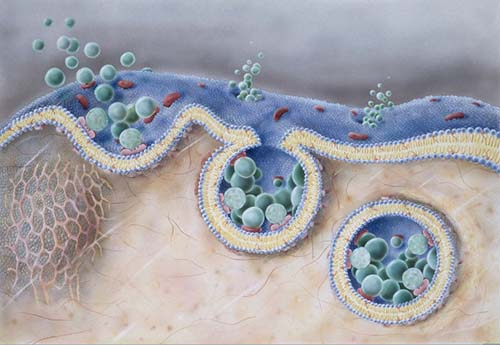
Endocytosis is a process that internalize molecules, mostly are large polar molecules, and turnover membrane components. One of the important protein in this process is called endophilin ,which is well-known in clathrin-denpendent endocytosis. Recently, Boucrot et al. demonstrated this protein also participates in a clathrin-independent endocytic pathway, as a central component. The article was published online in Nature.
The calthrin-independent endocytic pathway controlled by endophilin is called fast-acting tubulovesicular endocytic (FEME) pathway. The FEME is activated by ligand binding receptors which are recruited by interacting with SH3 domain of endophilin. Other two essential characteristics of endophilin are BAR domain, which act as membrane curvature effector, and multiple amphipathic helices, which enable to scissor membrane with the aid of dynamin. The recruitment of endophilin at plasma membrane is triggered by lamelliphdin, which in turn is mediated by PtdIns(3,4)P2, generated by SHIP1/2 from PtdIns(3,4,5)P3. In addition, the endophilin-mediated FEME pathway can be inhibited by inhibitors of multiple targets including dynamin, Rac, phosphatidylinositol-3-OH kinase, PAK1 and actin polymerization, and be activated by cargoes chalera, shiga toxins and IL-2R. The FEME pathway reveals a role of calthrin-independent pathway in the process of cell endocysis.
Reference:
Nature. 2014 Dec 17. 10.1038/nature14067.
Related Products
| Cat.No. | Product Name | Information |
|---|---|---|
| S2226 | Idelalisib (CAL-101) | Idelalisib (CAL-101) is a selective p110δ inhibitor with IC50 of 2.5 nM in cell-free assays; shown to have 40- to 300-fold greater selectivity for p110δ than p110α/β/γ, and 400- to 4000-fold more selectivity to p110δ than C2β, hVPS34, DNA-PK and mTOR. Idelalisib also stimulates autophagy. |
Related Targets
Selleck
Tech Support
Tel: +1-832-582-8158 Fax: +1-832-582-8590
Email: sales@selleckchem.com
Tel: +49 221 3579 1301
Email:eu.quote@selleckchem.com
Products are for research use only. Not for human use. We do not sell to patients.
©Copyright 2013 Selleck Chemicals. All Rights Reserved.
