- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Magnetic Separator
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Zotiraciclib
Synonyms: SB1317 (TG02)
Zotiraciclib (SB1317; TG02) is a novel small molecule potent CDK/JAK2/FLT3 inhibitor, emerged with potent CDK (IC50 against CDKs 1, 2 and 9 = 9, 5 and 3 nM, respectively), FLT3 (IC50 = 19 nM) and JAK2 (IC50 = 19 nM) potency.
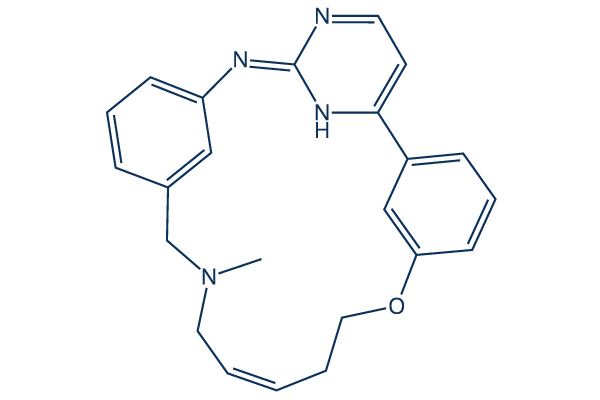
Zotiraciclib Chemical Structure
CAS No. 937270-47-8
Purity & Quality Control
Batch:
S700201
DMSO]15 mg/mL]false]Water]Insoluble]false]Ethanol]Insoluble]false
Purity:
99.98%
99.98
Zotiraciclib Related Products
Signaling Pathway
Biological Activity
| Description | Zotiraciclib (SB1317; TG02) is a novel small molecule potent CDK/JAK2/FLT3 inhibitor, emerged with potent CDK (IC50 against CDKs 1, 2 and 9 = 9, 5 and 3 nM, respectively), FLT3 (IC50 = 19 nM) and JAK2 (IC50 = 19 nM) potency. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | TG02 adopts a slightly different preferred conformation in order to achieve the key interaction with Asp698 when docks into FLT3. | ||||||||||
| Targets |
|
| In vitro | ||||
| In vitro | SB1317/TG02 inhibits the proliferating of all the cell lines tested including solid tumor cell lines such as colon (HCT-116, COLO205) and prostate (DU145) with IC50 of 33 nM, 72 nM and 140 nM, respectively. SB1317/TG02 potently inhibits the CDK2 biomarker pRb (phospho-Rb, retinoblastoma tumor suppressor protein) in HCT-116, and effects can be detected at the 40 nM with the protein phosphorylation being completely inhibited at 200 nM. SB1317/TG02 is potent against pRb in MV4-11 cells with IC50 of 0.13 μM and also inhibits pFLT3 and pSTAT5 in the same cell line. SB1317/TG02 results in the permeability (Papp) of 26h in the apical to basolateral (Papp,A→B) direction and in the basolateral to apical (Papp,B→A) direction of 28.0 × 10-6 cm/s and 27.4 ×10-6 cm/s, respectively, in the Caco-2 bidirectional permeability assays. SB1317/TG02 is found to be stable with a half-life of 45 min in human liver microsomes (HLM), is moderately stable in DLM (t1/2 = 33 min), and is quite rapidly cleared in MLM (t1/22 = 12 min) and in RLM (t1/2 = 11 min). [1] TG02 most potently inhibits CDK isoforms, inhibits CDK1, CDK2, CDK3, CDK5 and CDK9 with IC50 of 9 nM, 5 nM, 8 nM, 4 nM and 3 nM, respectively. TG02 also inhibits Lck, TYK2, Fyn, JAK2 and FLT3 with IC50 of 11 nM, 14 nM, 15 nM, 19 nM and 19 nM, respectively. TG02 has more potent anti-proliferative effects than SNS-032 in tumor cell lines. TG02 shows a stronger inhibition of the liquid tumor panel with IC50 of 0.13 μM compared with the solid tumor panel with IC50 of 0.30 μM. TG02 (100 nM) induces cell cycle arrest and apoptosis in MV4-11 cells. TG02 inhibits pRb, pFLT3 and pSTAT5 with IC50 of 125 nM, 4.7 μM and 560 nM, respectively, in MV4-11 cells. TG02 inhibits pJAK2 (Y1007/8) and pSTAT3 with IC50 of 63 nM and 53 nM, respectively, in Karpas 1106P. TG02 (300 nM) exposure leads to CDK9 inhibition, followed by G1 phase arrest and apoptotic induction, in HL-60 cells. [2] |
|||
|---|---|---|---|---|
| Kinase Assay | Enzyme Assays | |||
| All assays are carried out in 384-well white microtiter plates using the PKLight assay system. TG02 are tested at eight concentrations prepared from 3- or 4-fold serial dilution starting at 10 μM. For CDK2/cyclin A assay, the reaction mixture consist of the following components in 25 μL of assay buffer (50 mM Hepes, pH 7.5, 10 mM MgCl2, 5 mM MnCl2, 5 mM BGP, 1 mM DTT, 0.1 mM sodium orthovanadate), 1.4 μg/mL of CDK2/cyclin A complex, 0.5 μM RbING substrate, and 0.5 μM ATP. The mixture is incubated at room temperature for 2 hours. Then 13 μL of PKLight ATP detection reagent is added and the mixture is incubated for 10 min. Luminescence signals are detected on a multilabel plate reader. The other kinase assays are similar, with the following differences in reagents: For FLT3 assays, the mixture contains 2.0 μg/mL FLT3 enzyme, 5 μM poly(Glu,Tyr) substrate, and 4 μM ATP. For JAK2 assays, the reaction contains 0.35 μg/mL JAK2 enzyme, 10 μM poly(Glu,Ala,Tyr) substrate, and 0.15 μM ATP. The analytical software Prism 5.0 is used to generate IC50 values from the data. | ||||
| Cell Research | Cell lines | HCT-116, COLO205 and DU145 cell lines | ||
| Concentrations | ~10 μM | |||
| Incubation Time | 48 hours | |||
| Method | For proliferation assays in 96-well plates, 2×105 cells are seeded in 100 μL of medium and treated the following day with TG02 (in triplicate) at concentrations up to 10 μM for 48 hours. Cell viability is monitored using the CellTiter-96 Aqueous One solution cell proliferation assay. Dose-response curves are plotted to determine IC50 values for TG02 using the XL-fit software. |
|||
| In Vivo | ||
| In vivo | SB1317/TG02 is highly bound to plasma proteins in human, mouse, and dog plasma with PPB ranging between 99.4% to 99.9%. SB1317/TG02 shows oral bioavailability (F) of 4% and 37% in rats and dogs, respectively. SB1317/TG02 (75 mg/kg, orally) shows rapid absorption (tmax = 0.5 h) and a mean Cmax and AUC of 1029 ng/mL and 2523 ng•h/mL, respectively, with a mean terminal half-life of 6.1 hours, and oral bioavailability of 24% in mice. SB1317/TG02 (75 mg/kg po q.d. 3×/week) significantly inhibits the growth of tumors with a mean TGI of 82% in a murine sc xenograft model of human colon cancer (HCT-116), while the lower dose of 50 mg/kg po 3×/week is marginally effective. SB1317/TG02 (75 mg/kg po, 15 mg/kg ip) significantly inhibits the growth of tumors with mean TGIs of 42% and 63% for the oral and ip delivery methods, respectively, in a murine sc xenograft model of human B-cell lymphoma (Ramos). [1] TG02 (60 mg/kg) is selectively retained at supra-therapeutic levels and effectively inhibits CDK2, CDK9 and FLT3 in vivo, causing apoptosis in the tumor tissues, in tumor tissues in MV4-11 tumor-bearing nude mice. TG02 (10 mg/kg, 20 mg/kg and 40 mg/kg) leads to a tumor growth inhibition of 53%, 61% and 113%, respectively, in MV4-11 tumor-bearing nude mice. [2] |
|
|---|---|---|
| Animal Research | Animal Models | Murine sc xenograft model of human colon cancer (HCT-116) |
| Dosages | 75 mg/kg | |
| Administration | orally | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05588141 | Recruiting | Brain Tumor|Cancer |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
May 16 2023 | Phase 1|Phase 2 |
| NCT02942264 | Completed | Brain Tumor|Astrocytoma|Astroglioma|Glioblastoma|Gliosarcoma |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
December 14 2016 | Phase 1|Phase 2 |
| NCT01204164 | Completed | AML|ALL|Blast Crisis|MDS|Multiple Myeloma |
Tragara Pharmaceuticals Inc. |
August 2010 | Phase 1 |
Chemical Information & Solubility
| Molecular Weight | 372.46 | Formula | C23H24N4O |
| CAS No. | 937270-47-8 | SDF | -- |
| Smiles | CN1C/C=C/CCOC2=CC(=CC=C2)C3=CC=NC(=NC4=CC=CC(=C4)C1)N3 | ||
| Storage (From the date of receipt) | 3 years-21°C powder | ||
|
In vitro |
DMSO : 15 mg/mL ( (40.27 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Zotiraciclib | Zotiraciclib supplier | purchase Zotiraciclib | Zotiraciclib cost | Zotiraciclib manufacturer | order Zotiraciclib | Zotiraciclib distributor