- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Magnetic Separator
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Benfotiamine
Synonyms: S-Benzoylthiamine O-monophosphate, Benzoylthiamine monophosphate
Benfotiamine (S-Benzoylthiamine O-monophosphate) is a synthetic S-acyl derivative of thiamine (vitamin B1) and has been investigated for the treatment and prevention of Diabetic Nephropathy and Diabetes Mellitus, Type 2. Benfotiamine suppresses oxidative stress-induced NF-κB activation and prevents the bacterial endotoxin-induced inflammation.
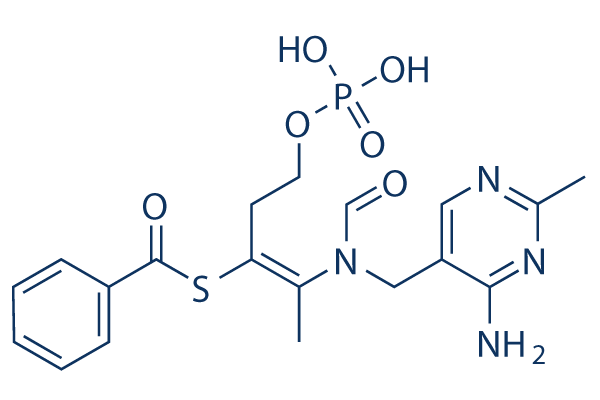
Benfotiamine Chemical Structure
CAS No. 22457-89-2
Purity & Quality Control
Batch:
Purity:
99.77%
99.77
Benfotiamine Related Products
Signaling Pathway
Biological Activity
| Description | Benfotiamine (S-Benzoylthiamine O-monophosphate) is a synthetic S-acyl derivative of thiamine (vitamin B1) and has been investigated for the treatment and prevention of Diabetic Nephropathy and Diabetes Mellitus, Type 2. Benfotiamine suppresses oxidative stress-induced NF-κB activation and prevents the bacterial endotoxin-induced inflammation. | |
|---|---|---|
| Targets |
|
| In vitro | ||||
| In vitro | Benfotiamine improves the expression of endothelial cell markers in EPCs, restores eNOS levels, and recovers the ability of EPCs to participate in angiogenic processes. It is able to dampen glucose toxicity effects on endothelial progenitors[1]. Benfotiamine possesses antitumor activity against leukemia cells. In a panel of nine myeloid leukemia cell lines benfotiamine impairs the viability of HL-60, NB4, K562 and KG1 cells and also inhibits the growing of primary leukemic blasts. The antitumor activity of benfotiamine is not mediated by apoptosis, necrosis or autophagy, but rather occurs though paraptosis cell death induction. Benfotiamine inhibits the activity of constitutively active ERK1/2 and concomitantly increases the phosphorylation of JNK1/2 kinase in leukemic cells. In addition, benfotiamine induces the down regulation of the cell cycle regulator CDK3 which results in G1 cell cycle arrest in the sensitive leukemic cells[2]. |
|||
|---|---|---|---|---|
| Cell Research | Cell lines | leukemia cells (HL60, AML 1, NB4 cells) | ||
| Concentrations | 50 μM | |||
| Incubation Time | 24, 48, 72, 96 h | |||
| Method | Cell viability is assessed using a colorimetric MTT metabolic activity assay. |
|||
| In Vivo | ||
| In vivo | Benfotiamine might exert vascular and renal benefits by modulating mechanisms independent or downstream of ROS formation. Benfotiamine aids the post-ischaemic healing of diabetic animals via PKB/Akt-mediated potentiation of angiogenesis and inhibition of apoptosis[3]. |
|
|---|---|---|
| Animal Research | Animal Models | diabetic animal model induced by STZ (male CD1 mice) |
| Dosages | 80 mg/kg | |
| Administration | oral administration | |
Chemical Information & Solubility
| Molecular Weight | 466.45 | Formula | C19H23N4O6PS |
| CAS No. | 22457-89-2 | SDF | -- |
| Smiles | CC1=NC=C(C(=N1)N)CN(C=O)C(=C(CCOP(=O)(O)O)SC(=O)C2=CC=CC=C2)C | ||
| Storage (From the date of receipt) | |||
|
In vitro |
5%TFA : 6 mg/mL |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Benfotiamine | Benfotiamine supplier | purchase Benfotiamine | Benfotiamine cost | Benfotiamine manufacturer | order Benfotiamine | Benfotiamine distributor