research use only
Arbidol HCl Influenza Virus inhibitor
Cat.No.S2120
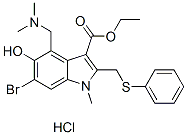
Chemical Structure
Molecular Weight: 513.88
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| Other Influenza Virus Inhibitors | Nucleozin (-)-Arctigenin Chebulinic acid Adamantane D-Pinitol Cetylpyridinium chloride monohydrate |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(194.59 mM)
Ethanol : 50 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 513.88 | Formula | C22H25BrN2O3S.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 131707-23-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Umifenovir hydrochloride, Arbidol hydrochloride | Smiles | CCOC(=O)C1=C(N(C2=CC(=C(C(=C21)CN(C)C)O)Br)C)CSC3=CC=CC=C3.Cl | ||
Mechanism of Action
| In vitro |
Arbidol inhibits the cell entry of HCV pseudoparticles of genotypes 1a, 1b, and 2a in a dose-dependent fashion. Arbidol also displays a dose-dependent inhibition of HCV membrane fusion, as assayed by using HCV pseudoparticles (HCVpp) and fluorescent liposomes. Arbidol is found to present potent inhibitory activity against enveloped and non-enveloped RNA viruses, including FLU-A, RSV, HRV 14 and CVB3 when added before, during, or after viral infection, with IC50 ranging from 2.7 to 13.8 mg/mL. Arbidol shows selective antiviral activity against AdV-7, a DNA virus, only when added after infection (therapeutic index (TI) = 5.5). Arbidol induces changes to viral mRNA synthesis of the PB2, PA, NP, NA, and NS genes in MDCK cultures infected with influenza A/PR/8/34. Arbidol interacts and modifies the physicochemical properties of the phospholipids in the membrane, having a significant effect on negatively charged phospholipids but a minor one on zwitterionic phospholipids. Arbidol is located at the interface of the membrane, participates in hydrogen bonding either with water or the phospholipid or both, and decreases the hydrogen bonding network of the phospholipids giving place to a phospholipid phase similar to the dehydrated solid one. Arbidol is found to have potent inhibitory activity against HTNV when added in vitro before or after viral infection, with IC50 of 0.9 mg/mL and 1.2 mg/mL, respectively.
|
References |
|
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01651663 | Unknown status | Influenza |
Pharmstandard |
September 2011 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































