- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Ceralasertib (AZD6738) ATR inhibitor
Cat.No.S7693

Chemical Structure
Molecular Weight: 412.51
Jump to
Quality Control
Batch:
Purity:
99.51%
99.51
Products Often Used Together with Ceralasertib (AZD6738)
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| breast cancer cell lines | Cell growth inhibition assay | 0.125, 0.25, 0.5 and 1.0 μM | 5 days | IC50 values ranged from 0.3 to >1 μmol/L | 27501113 | |
| SNU-601 cells | Cell growth inhibition assay | 0-1 μmol/L | 5 days | The S and sub-G1 populations of SNU-601 cells were dramatically and dose-dependently increased by AZD6738. | 28138034 | |
| K8484 cells | Function assay | 2 μM | 7 hours | In K8484 cells, AZD6738 at 2 µM completely prevented LY-188011-induced Chk1 phosphorylation on Serine 345, the downstream ATR target. | 29891488 | |
| LICR-LON-HN4 and LICR-LON-HN5 cells | Function assay | 0.03, 0.1, 0.3, 1, 3, 10 μM | AZD6738 inhibition of ATR through loss of downstream phosphorylation of CHK1 on Ser345. | 30057890 | ||
| LoVo cells | Function assay | 24 h | Reduction in cell count; a proportion of the cell population are (in addition to cell cycle arrest) undergoing apoptosis when exposed to drug at concentrations greater than 3 μM | 26310312 | ||
| HT29 cells | Function assay | 60 mins | IC50 = 0.074 μM | 30346772 | ||
| LoVo cells | Cytotoxicity assay | 72 hrs | GI50 = 0.44 μM | 30346772 | ||
| LoVo cells | Function assay | 25 mg/kg | 8 hrs | Cp = 0.74 μM | 30346772 | |
| LoVo cells | Function assay | 50 mg/kg | 8 hrs | Cp = 2.2 μM | 30346772 | |
| HT-29 cells | Cytotoxicity assay | 72 hrs | GI50 = 2.6 μM | 30346772 | ||
| LoVo cells | Function assay | 75 mg/kg | 8 hrs | Cp = 2.6 μM | 30346772 | |
| MDA-MB-468 cells | Function assay | IC50 = 5.7 μM | 30346772 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 82 mg/mL
(198.78 mM)
Ethanol : 5 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 412.51 | Formula | C20H24N6O2S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1352226-88-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1COCCN1C2=NC(=NC(=C2)C3(CC3)S(=N)(=O)C)C4=C5C=CNC5=NC=C4 | ||
Mechanism of Action
| Targets/IC50/Ki |
ATR
(Cell-free assay) 1 nM
|
|---|---|
| In vitro |
In four Kras mutant cell lines: H23, H460, A549, and H358, Ceralasertib (AZD6738) inhibits ATR kinase activity and impairs cell viability. In ATM-deficient H23 cells, it strongly synergizes with NSC 119875 to induce rapid cell death. In p53 or ATM defective cells, this compound treatment results in replication fork stalls and accumulation of unrepaired DNA damage, resulting in cell death by mitotic catastrophe. |
| In vivo |
In nude mice bearing H460 and H23 tumors, Ceralasertib (AZD6738) (50 mg/kg, p.o.) results in tumor growth inhibition (TGI), and its combination with NSC 119875 causes rapid regression of ATM-deficient H23 tumors. In nude mice bearing LoVo xenografts, a combination of this compound (50 mg/kg) + IR (2 Gy) avoids toxicity while still maintaining efficacy. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pCHK1 / pCDC25c / pRPA32 / γH2AX / pHH3 / cleaved caspase-3 / RAD51 ATM pSer1981 / ATM / ATR / Chk1 pSer345 / Chk1 / Chk2 pThr68 / Chk2 |
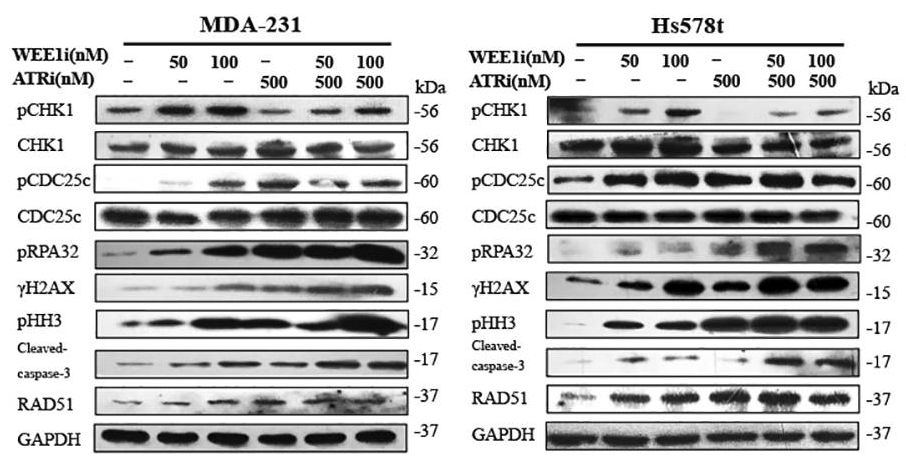
|
29605721 |
| Immunofluorescence | γH2AX / RAD51 53BP1 |
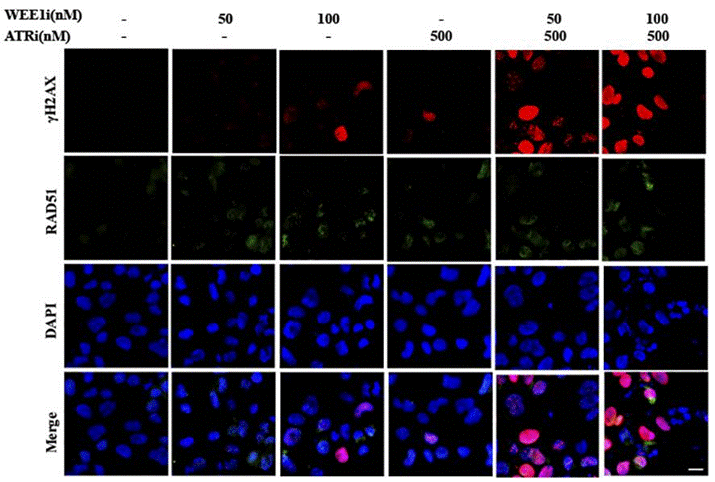
|
29605721 |
| Growth inhibition assay | Cell viability IC50 |
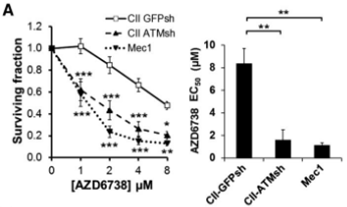
|
26563132 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05941897 | Active not recruiting | Advanced or Metastatic NSCLC |
AstraZeneca |
June 21 2023 | Phase 2 |
| NCT05514132 | Active not recruiting | Advanced Solid Tumours |
AstraZeneca |
September 23 2022 | Phase 1 |
| NCT05450692 | Recruiting | Advanced or Metastatic Non-Small Cell Lung Cancer |
AstraZeneca|Parexel |
September 15 2022 | Phase 3 |
| NCT05061134 | Active not recruiting | Melanoma |
AstraZeneca |
August 11 2022 | Phase 2 |
| NCT05469919 | Active not recruiting | Advanced Solid Malignancies |
AstraZeneca |
June 9 2022 | Phase 1 |
| NCT04704661 | Recruiting | Advanced Breast Carcinoma|Advanced Colon Carcinoma|Advanced Colorectal Carcinoma|Advanced Endometrial Carcinoma|Advanced Gastric Carcinoma|Advanced Gastroesophageal Junction Adenocarcinoma|Advanced Malignant Solid Neoplasm|Advanced Salivary Gland Carcinoma|Anatomic Stage III Breast Cancer AJCC v8|Anatomic Stage IIIA Breast Cancer AJCC v8|Anatomic Stage IIIB Breast Cancer AJCC v8|Anatomic Stage IIIC Breast Cancer AJCC v8|Anatomic Stage IV Breast Cancer AJCC v8|Clinical Stage III Gastric Cancer AJCC v8|Clinical Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IV Gastric Cancer AJCC v8|Clinical Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IVA Gastric Cancer AJCC v8|Clinical Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IVB Gastric Cancer AJCC v8|Clinical Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8|HER2-Positive Breast Carcinoma|Malignant Hepatobiliary Neoplasm|Metastatic Breast Carcinoma|Metastatic Gastroesophageal Junction Adenocarcinoma|Metastatic Malignant Solid Neoplasm|Pathologic Stage III Gastric Cancer AJCC v8|Pathologic Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIA Gastric Cancer AJCC v8|Pathologic Stage IIIA Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIB Gastric Cancer AJCC v8|Pathologic Stage IIIB Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIC Gastric Cancer AJCC v8|Pathologic Stage IV Gastric Cancer AJCC v8|Pathologic Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8|Prognostic Stage III Breast Cancer AJCC v8|Prognostic Stage IIIA Breast Cancer AJCC v8|Prognostic Stage IIIB Breast Cancer AJCC v8|Prognostic Stage IIIC Breast Cancer AJCC v8|Prognostic Stage IV Breast Cancer AJCC v8|Stage III Colon Cancer AJCC v8|Stage III Colorectal Cancer AJCC v8|Stage III Major Salivary Gland Cancer AJCC v8|Stage III Uterine Corpus Cancer AJCC v8|Stage IIIA Colon Cancer AJCC v8|Stage IIIA Colorectal Cancer AJCC v8|Stage IIIA Uterine Corpus Cancer AJCC v8|Stage IIIB Colon Cancer AJCC v8|Stage IIIB Colorectal Cancer AJCC v8|Stage IIIB Uterine Corpus Cancer AJCC v8|Stage IIIC Colon Cancer AJCC v8|Stage IIIC Colorectal Cancer AJCC v8|Stage IIIC Uterine Corpus Cancer AJCC v8|Stage IIIC1 Uterine Corpus Cancer AJCC v8|Stage IIIC2 Uterine Corpus Cancer AJCC v8|Stage IV Colon Cancer AJCC v8|Stage IV Colorectal Cancer AJCC v8|Stage IV Major Salivary Gland Cancer AJCC v8|Stage IV Uterine Corpus Cancer AJCC v8|Stage IVA Colon Cancer AJCC v8|Stage IVA Colorectal Cancer AJCC v8|Stage IVA Major Salivary Gland Cancer AJCC v8|Stage IVA Uterine Corpus Cancer AJCC v8|Stage IVB Colon Cancer AJCC v8|Stage IVB Colorectal Cancer AJCC v8|Stage IVB Major Salivary Gland Cancer AJCC v8|Stage IVB Uterine Corpus Cancer AJCC v8|Stage IVC Colon Cancer AJCC v8|Stage IVC Colorectal Cancer AJCC v8|Stage IVC Major Salivary Gland Cancer AJCC v8|Unresectable Colorectal Carcinoma|Unresectable Gastroesophageal Junction Adenocarcinoma|Unresectable Malignant Solid Neoplasm |
National Cancer Institute (NCI) |
August 9 2021 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































