|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C25H34N4O2.C4H4O4 |
|||
| Molecular Weight | 538.64 | CAS No. | 1092679-51-0 | |
| Solubility (25°C)* | In vitro | DMSO | 85 mg/mL (157.8 mM) | |
| Ethanol | 85 mg/mL (157.8 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | WAY-100635 Maleate is a potent and selective 5-HT receptor antagonist with IC50 of 0.95 nM. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | In dorsal raphe nucleus (DRN) slices superfused with WAY 100635 (10 nM), the majority of putative 5-HT neurons increase their firing rate (13% of baseline rate). In addition, WAY 100635 completely prevents the decrease in firing rate produced by 5-HT (3-15 μM), 8-OH-DPAT (10 nM), 5-carboxamidotryptamine (20 nM) and lesopitron (100 nM). The antagonism exerted by WAY 100635 is fully surmounted by increasing the concentration of 5-HT to 300 μM with an IC50 of 0.95 nM. In hippocampal slices, WAY 100635 (0.5 nM -10 nM) does not alter the resting membrane potential or the membrane input resistance of intracellularly recorded CA1 pyramidal cells. However, WAY 100635 completely prevents not only the hyperpolarization, with an IC50 of 1.3 nM, but also the decrease in membrane input resistance produced by 5-HT and 5-carboxamidotryptamine with IC50 of 22.5 μM and 50 nM, respectively. [1] WAY 100635 has an IC50 of 1.35 nM and is > 100-fold selective for the 5-HT1A site relative to a range of other CNS receptors. The Bmax of [3H]WAY 100635 specific binding is consistently 50-60% greater than that of the agonist radioligand, [3H]8-OH-DPAT. Mn2+, but not guanine nucleotides, inhibits [3H]WAY 100635-specific binding. WAY 100635 has no 5-HT1A receptor agonist actions, but dose-dependently blocks the effects of agonists at both the postsynaptic 5-HT1A receptor in the CA1 region of the hippocampus, and the somatodendritic 5-HT1A receptor locates on dorsal raphe 5-HT neurones. [2] [3H]WAY 100635 has a Kd of approximately 2.5 nM. [3] In the isolated guinea-pig ileum WAY 100635 is a potent and, at high concentrations, an insurmountable antagonist of the 5-HT1A receptor agonist action of 5-carboxamidotryptamine, with an apparent pA2 value (at 0.3 nM) of 9.71. [4] Five minutes after the i.v. injection of [3H]WAY 100635 (4 μCi -7.6 μCi per mouse) the amount of tritium found in the whole brain only accounted for 1.5-1.8% of the injected radioactivity, regional differences in 3H accumulation already correspondes to those of 5-HT1A receptor density. [5] In light of its only recently discovered dopaminergic activity, conclusions drawn from studies that employs WAY 100635 as a selective 5-HT1A antagonist may need to be re-evaluated. [6] | ||
| In vivo | [3H]WAY 100635 is shown to bind selectively to brain 5-HT1A receptors, following intravenous administration to mice. WAY 100635 also dose-dependently blocks the ability of 8-OH-DPAT to inhibit the firing of dorsal raphe 5-HT neurones, and to induce the '5-HT syndrome', hypothermia, hyperphagia and to elevate plasma ACTH levels. In the mouse light/dark box anxiety model, WAY 100635 induces anxiolytic-like effects. WAY 100635 has no intrinsic effect on cognition in the delayed-matching-to-position model of short-term memory in the rat, but reverses the disruptive effects of 8-OH-DPAT on motor motivational performance. [2] WAY 100635 blocks the inhibitory action of 8-OH-DPAT on dorsal raphe neuronal firing in the anaesthetised rat at doses which has no inhibitory action per se. In behavioural models, WAY 100635 itself induces no overt behavioural changes but potently antagonises the behavioural syndrome induced by 8-OH-DPAT in the rat and guinea-pig (minimum effective dose = 0.003 mg/kg s.c. and ID50 = 0.01 mg/kg s.c., respectively). WAY 100635 also blocks the hypothermia induced by 8-OH-DPAT in the mouse and rat with ID50 values of 0.01 mg/kg s.c. [4] | ||
| Features | Characterised as the first 5-HT1A antagonist radioligand. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
-
, , Sci Rep, 2014, 4:7270.
-
Data from [Data independently produced by , , Neurochem Res, 2018, 43(7):1371-1382]
Selleck's WAY-100635 Maleate has been cited by 8 publications
| Targeting 5-Hydroxytryptamine Receptor 1A in the Portal Vein to Decrease Portal Hypertension [ Gastroenterology, 2024, 167(5):993-1007] | PubMed: 38906512 |
| Regular Aerobic Exercise Attenuates Pain and Anxiety in Mice by Restoring Serotonin-Modulated Synaptic Plasticity in the Anterior Cingulate Cortex [ Med Sci Sports Exerc, 2022, 54(4):566-581] | PubMed: 34935710 |
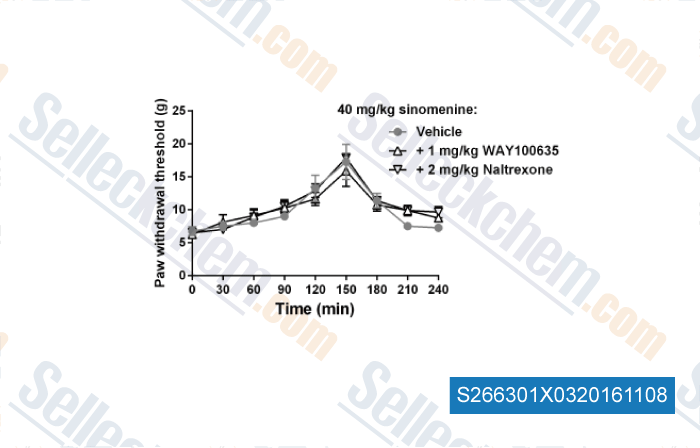
| N-Demethylsinomenine, an active metabolite of sinomenine, attenuates chronic neuropathic and inflammatory pain in mice [ Sci Rep, 2021, 11(1):9300] | PubMed: 33927244 |
| Spinal Serotonin 1A Receptor Contributes to the Analgesia of Acupoint Catgut Embedding by Inhibiting Phosphorylation of the N-Methyl-d-Aspartate Receptor GluN1 Subunit in Complete Freund's Adjuvant-Induced Inflammatory Pain in Rats [Cui WQ, et al. J Pain, 2019, 20(1):16.e1-16.e16] | PubMed: 30102991 |
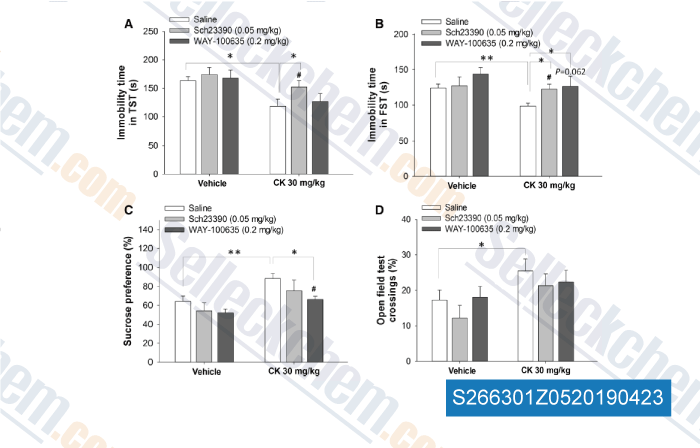
| Antidepressant Effects of the Ginsenoside Metabolite Compound K, Assessed by Behavioral Despair Test and Chronic Unpredictable Mild Stress Model [Song W, et al. Neurochem Res, 2018, 43(7):1371-1382] | PubMed: 29790069 |
| Levo-Tetrahydroberberrubine Produces Anxiolytic-Like Effects in Mice through the 5-HT1A Receptor. [Mi G, et al. PLoS One, 2017, 12(1):e0168964] | PubMed: 28085967 |
| Stimulation of Anxiety-Like Behavior via ERK Pathway by Competitive Serotonin Receptors 2A and 1A in Post-Traumatic Stress Disordered Mice. [ Neurosignals, 2017, 25(1):39-53] | PubMed: 28977803 |
| Antinociceptive effects of sinomenine in a rat model of neuropathic pain. [Zhu Q, et al. Sci Rep, 2014, 4:7270] | PubMed: 25434829 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
