|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C9H12ClN |
||||||||||
| Molecular Weight | 169.65 | CAS No. | 1986-47-6 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 34 mg/mL (200.41 mM) | ||||||||
| Water | 34 mg/mL (200.41 mM) | ||||||||||
| Ethanol | 34 mg/mL (200.41 mM) | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Tranylcypromine HCl is a monoamine oxidase inhibitor, which inhibits CYP2A6 with Ki of 0.08 μM and 0.2 μM in cDNA-expressing microsomes and Human Liver Microsomes, respectively. | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
||||||
| In vitro | R-(+)-Tranylcypromine, (±)-Tranylcypromine, and S-(−)-Tranylcypromine competitively inhibits CYP2A6-mediated metabolism of nicotine with apparent Ki values of 0.05 μM, 0.08 μM, and 2.0 μM, respectively, in human liver microsomes. [1] Tranylcypromine (500 μg/mL) severely inhibits bradykinin-stimulated arachidonic acid release in calf aortic endothelial cells. [2] Tranylcypromine inhibits CYP2A6 and CYP2E1 activity with IC50 of 0.42 μM and 3 μM, respectively, in human liver microsomes. Tranylcypromine induces type II and cyclopropylbenzene type I difference spectrum in human liver microsomes. [3] | ||||||
| In vivo | Tranylcypromine injection leads to a large, slow increase in motor activity at 5 mg/kg and 10 mg/kg compared with the saline control in male rats, although 2 mg/kg Tranylcypromine does not induce an increase. Tranylcypromine (10 mg/kg) also causes significant decreases in the number of rearing behaviors in male rats. [4] |
Protocol (from reference)
References
Customer Product Validation
-
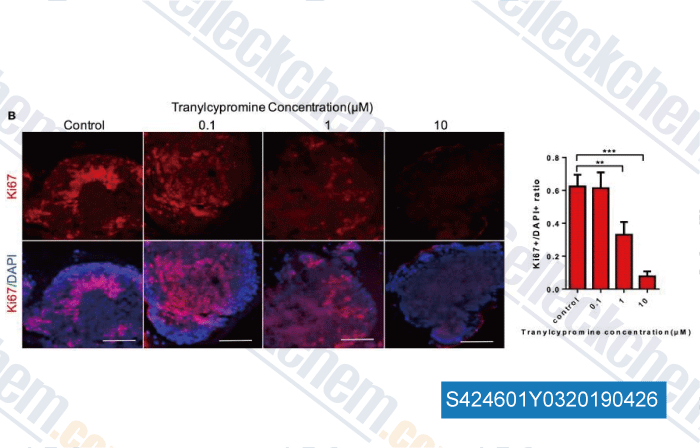
Data from [Data independently produced by , , Front Neurol, 2017, 8:626]
Selleck's Tranylcypromine HCl has been cited by 20 publications
| Restoring sweat gland function in mice using regenerative sweat gland cells derived from chemically reprogrammed human epidermal keratinocytes [ Sci Bull (Beijing), 2024, S2095-9273(24)00799-0] | PubMed: 39550273 |
| Transcriptome-based chemical screens identify CDK8 as a common barrier in multiple cell reprogramming systems [ Cell Rep, 2023, 42(6):112566] | PubMed: 37235474 |
| Kawain Inhibits Urinary Bladder Carcinogenesis through Epigenetic Inhibition of LSD1 and Upregulation of H3K4 Methylation [ Biomolecules, 2023, 13(3)521] | PubMed: 36979456 |
| Kawain Inhibits Urinary Bladder Carcinogenesis through Epigenetic Inhibition of LSD1 and Upregulation of H3K4 Methylation [ Biomolecules, 2023, 13(3)521] | PubMed: 36979456 |
| Novel dual LSD1/HDAC6 inhibitor for the treatment of cancer [ PLoS One, 2023, 18(1):e0279063] | PubMed: 36595522 |
| Novel dual LSD1/HDAC6 inhibitor for the treatment of cancer [ PLoS One, 2023, 18(1):e0279063] | PubMed: 36595522 |
| Direct chemical reprogramming of human cord blood erythroblasts to induced megakaryocytes that produce platelets [ Cell Stem Cell, 2022, 29(8):1229-1245.e7] | PubMed: 35931032 |
| Direct Reprograming of Mouse Fibroblasts into Dermal Papilla Cells via Small Molecules [ Int J Mol Sci, 2022, 23(8)4213] | PubMed: 35457029 |
| Epigenetic Dysregulation Induces Translocation of Histone H3 into Cytoplasm [ Adv Sci (Weinh), 2021, e2100779] | PubMed: 34363353 |
| LSD1-mediated demethylation of OCT4 safeguards pluripotent stem cells by maintaining the transcription of PORE-motif-containing genes [ Sci Rep, 2021, 11(1):10285] | PubMed: 33986438 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
