|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C12H9F3N2O2 |
|||
| Molecular Weight | 270.21 | CAS No. | 163451-81-8 | |
| Solubility (25°C)* | In vitro | DMSO | 54 mg/mL (199.84 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Teriflunomide (A77 1726, HMR-1726) is the active metabolite of leflunomide, inhibiting pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase, used as an immunomodulatory agent. | |
|---|---|---|
| Targets |
|
|
| In vitro | Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzyme involved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high-avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus, teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes. [1] | |
| In vivo | Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological, and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity. [1] |
Protocol (from reference)
Customer Product Validation
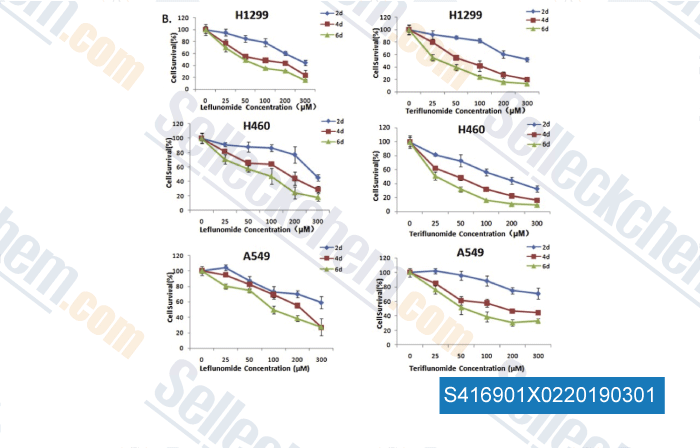
-
Data from [Data independently produced by , , Toxicol Lett, 2018, 282:154-165]
Selleck's Teriflunomide has been cited by 20 publications
| Selective vulnerability of human-induced pluripotent stem cells to dihydroorotate dehydrogenase inhibition during mesenchymal stem/stromal cell purification [ Front Cell Dev Biol, 2023, 11:1089945] | PubMed: 36814599 |
| Proof-of-principle studies on a strategy to enhance nucleotide imbalance specifically in cancer cells [ Cell Death Discov, 2022, 8(1):464] | PubMed: 36424385 |
| Inhibitors of dihydroorotate dehydrogenase cooperate with molnupiravir and N4-hydroxycytidine to suppress SARS-CoV-2 replication [ iScience, 2022, 25(5):104293] | PubMed: 35492218 |
| Inhibition of de novo pyrimidine synthesis augments Gemcitabine induced growth inhibition in an immunocompetent model of pancreatic cancer [ Int J Biol Sci, 2021, 17(9):2240-2251] | PubMed: 34239352 |
| The Mitochondria-Independent Cytotoxic Effect of Leflunomide on RPMI-8226 Multiple Myeloma Cell Line [ Molecules, 2021, 26(18)5653] | PubMed: 34577124 |
| Inhibition of canine distemper virus replication by blocking pyrimidine nucleotide synthesis with A77 1726, the active metabolite of the anti-inflammatory drug leflunomide [ J Gen Virol, 2021, 102(3)] | PubMed: 33416466 |
| Application of a newly-developed cynomolgus macaque BiTE-mediated cytotoxic T-lymphocyte activity assay to various immunomodulatory agents in vitro [ J Immunotoxicol, 2021, 18(1):154-162] | PubMed: 34714999 |
| N4-hydroxycytidine and inhibitors of dihydroorotate dehydrogenase synergistically suppress SARS-CoV-2 replication [ bioRxiv, 2021, 10.1101/2021.06.28.450163] | PubMed: N/A |
| N4-hydroxycytidine and inhibitors of dihydroorotate dehydrogenase synergistically suppress SARS-CoV-2 replication [ bioRxiv, 2021, 10.1101/2021.06.28.450163] | PubMed: None |
| Actionable Cytopathogenic Host Responses of Human Alveolar Type 2 Cells to SARS-CoV-2 [ Mol Cell, 2020, 80(6):1104-1122.e9] | PubMed: 33259812 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
