|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C13H20N2O2.HCl |
|||
| Molecular Weight | 272.77 | CAS No. | 51-05-8 | |
| Solubility (25°C)* | In vitro | DMSO | 55 mg/mL (201.63 mM) | |
| Water | 55 mg/mL (201.63 mM) | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Procaine (Novocaine) is an inhibitor of sodium channel, NMDA receptor and nAChR with IC50 of 60 μM, 0.296 mM and 45.5 μM, which is also an inhibitor of 5-HT3 with KD of 1.7 μM. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||
| In vitro | Procaine acts mainly by inhibiting sodium influx through voltage gated sodium channels in the neuronal cell membrane of peripheral nerves. When the influx of sodium is interrupted, an action potential cannot arise and signal conduction is thus inhibited. The receptor site is thought to be located at the cytoplasmic (inner) portion of the sodium channel. [1] Procaine has also been shown to bind or antagonize the function of N-methyl-D-aspartate (NMDA) receptors [2] as well as nicotinic acetylcholine receptors [3] and the serotonin receptor-ion channel complex. [4] Procaine is an inhibitor of the mechanisms of Ca-induced Ca release and caffeine-induced Ca release in various types of muscle preparations. 0.5 mM Procaine blocks individual sarcoplasmic reticulum Ca2+ release channels in planarlipid bilayers. Procaine does not reduce the single channel conductance nor appreciably shorts the mean open times of the channel, rather, it increases the longest closed time. [5] Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. 0.5 mM Procaine produces a 40% reduction in 5-methylcytosine DNA in MCF-7 breast cancer cell line. Procaine can also bind to CpG-enriched DNA, and demethylates densely hypermethylated CpG islands, leading to restoring gene expression of epigenetically silenced genes. Procaine treatment (0.5 mM) induces an increase in the mitotic index of cells in M phase. Procaine treatment (1 mM) reduces cell proliferation by ~40%. [6] Procaine influences red cell shape and deformability. 45 mM Procaine almost completely prevents the discocyte-echinocyte transformation associated with ATP depletion. Similar concentrations of Procaine normalize the viscosity and filterability, but have no effect on cell volume, osmotic fragility, or monovalent cation composition of cells undergoing ATP depletion. [7] | ||||||||
| In vivo | Procaine is an excitant of limbic system cells. 15 mg/kg Procaine increases cellular activity in amygdala ventral hippocampus, nucleus accumbens, temporal neocortex and ventromedial hypothalamus of awaken cat. Procaine facilitates transmission of evoked excitatory activity from the amygdala to the ventromedial hypothalamus. [8] Procaine influences frequency and amplitude of reticularly elicited hippocampal rhythmical slow activity. Procaine (0.5 μL, 20% wt/vol) injected at points in the ascending system anterior to the supramamillary nucleus, in the region of the medial forebrain bundle or in the medial septum, reduces the amplitude of reticularly elicited rhythmical slow activity (RSA) but has no effect on its frequency. Procaine injected at points in the ascending system from just anterior to the reticular formation stimulation site, up to, and including the supramamillary nucleus, reduces both the frequency and amplitude of reticularly elicited RSA. [9] Procaine (80mg/kg) increases the duration and propagation of epileptiform afterdischarges (ADs) produced by electrical stimulation of the amygdala in rats. Porcaine also increases the rate of seizure development (kindling) produced by repeated stimulation of the amygdala. Procaine would itself act as convulsants in well kindled subjects. Procaine produces a weak but significant increase in the amplitude of the transcallosal evoked potential. [10] Procaine influences generation of auditory brain stem responses (ABRs). Procaine (30 μL of 1% solution) injection into the trapezoid body of guinea pig affects many of the components of the scalp-derived ABR: N2 is delayed making P2 broader in duration, P3 and N3 are lost, P4 is shortened in latency, broadened in duration but unaffected in amplitude, and N4 is considerably attenuated. Only P1 and N1 are unaffected by the procaine injection. [11] Procaine increases the therapeutic index of cisplatin by improving antitumor activity of cisplatin and reducing its nephrotoxicity. Simultaneous administration of cisplatin and Procaine (40 mg/kg) to BDF1 mice produces 50% lethal dose (LD50) and 90% lethal dose (LD90) values approximately two times higher than those observed with cisplatin alone. Simultaneous administration produces a higher cure rates compared with cisplatin alone (50% vs 9%). The increased blood urea nitrogen (BUN) levels observed 4-7 days following a single administration of cisplatin, as well as the tubular degenerative changes detected by light microscopy, are not observed when the same doses of cisplatin are given simultaneously with Procaine. [12] |
Protocol (from reference)
References
Customer Product Validation
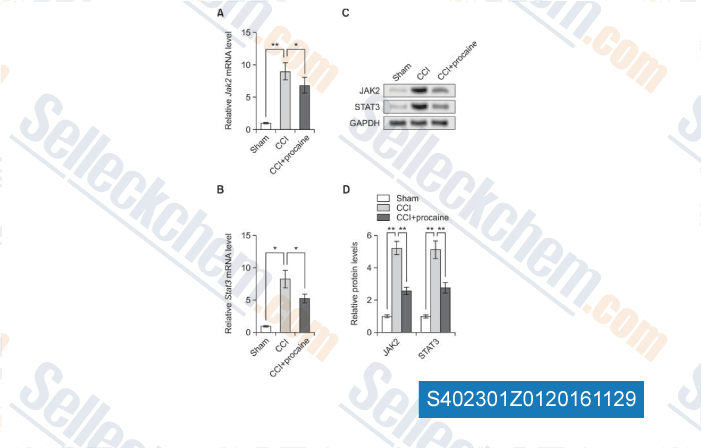
-
Data from [Data independently produced by , , Biomol Ther (Seoul), 2016, 24(5): 489-494.]
Selleck's Procaine HCl has been cited by 2 publications
| Procaine Attenuates Pain Behaviors of Neuropathic Pain Model Rats Possibly via Inhibiting JAK2/STAT3. [Li D, et al. Biomol Ther (Seoul), 2016, 24(5):489-94] | PubMed: 27530113 |
| Procaine Attenuates Pain Behaviors of Neuropathic Pain Model Rats Possibly via Inhibiting JAK2/STAT3. [Li D, et al. Biomol Ther (Seoul), 2016, 24(5):489-94] | PubMed: 27530113 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
