|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C19H19ClN6O2 |
|||
| Molecular Weight | 398.85 | CAS No. | 1297538-32-9 | |
| Solubility (25°C)* | In vitro | DMSO | 80 mg/mL (200.57 mM) | |
| Ethanol | 4 mg/mL (10.02 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Darolutamide (ODM-201, BAY-1841788) is a novel androgen receptor (AR) antagonist that blocks AR nuclear translocation with Ki of 11 nM. Phase 3. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | In AR-HEK293 cells stably expressing full-length hAR, ODM-201 inhibits human AR (hAR) with IC50 of 26 nM. ODM-201 inhibits VCaP cell proliferation with IC50 of 230 nM, while has no effect on the viability of AR-negative cell lines tested, DU-145 prostate cancer cells and H1581 lung cancer cells. [1] | ||
| In vivo | In mice bearing VCaP xenografts, ODM-201 (50 mg/kg, p.o.) significantly inhibits castration-resistant prostate tumor growth. [1] |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[1] |
|
| Animal Study:[1] |
|
Customer Product Validation
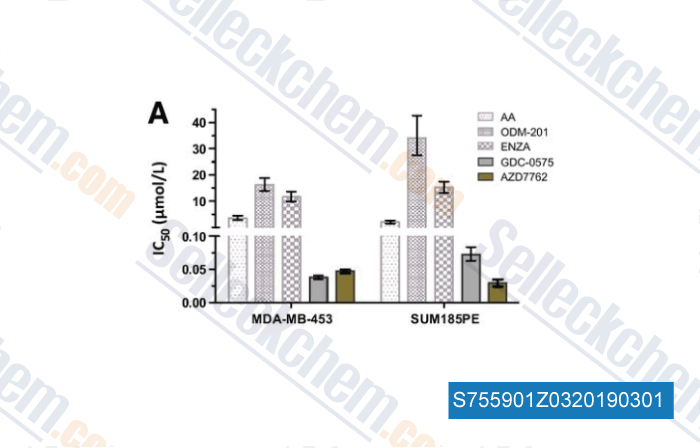
-
Data from [Data independently produced by , , Clin Cancer Res, 2018, doi:10.1158/1078-0432.CCR-18-1469]
Selleck's Darolutamide (ODM-201) has been cited by 16 publications
| Increased translation driven by non-canonical EZH2 creates a synthetic vulnerability in enzalutamide-resistant prostate cancer [ Nat Commun, 2024, 15(1):9755] | PubMed: 39567499 |
| Darolutamide-mediated phospholipid remodeling induces ferroptosis through the SREBP1-FASN axis in prostate cancer [ Int J Biol Sci, 2024, 20(12):4635-4653] | PubMed: 39309439 |
| Loss of LCMT1 and biased protein phosphatase 2A heterotrimerization drive prostate cancer progression and therapy resistance [ Nat Commun, 2023, 14(1):5253] | PubMed: 37644036 |
| Loss of LCMT1 and biased protein phosphatase 2A heterotrimerization drive prostate cancer progression and therapy resistance [ Nat Commun, 2023, 14(1):5253] | PubMed: 37644036 |
| Pharmacological Targeting of Androgen Receptor Elicits Context-Specific Effects in Estrogen Receptor-Positive Breast Cancer [ Cancer Res, 2023, 83(3):456-470] | PubMed: 36469363 |
| Allosteric inhibition of HSP70 in collaboration with STUB1 augments enzalutamide efficacy in antiandrogen resistant prostate tumor and patient-derived models [ Pharmacol Res, 2023, 189:106692] | PubMed: 36773708 |
| Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy [ Cell Metab, 2022, S1550-4131-2200411-9] | PubMed: 36257316 |
| A novel inhibitor of ARfl and ARv7 induces protein degradation to overcome enzalutamide resistance in advanced prostate cancer [ Acta Pharm Sin B, 2022, 12(11):4165-4179] | PubMed: 36386477 |
| An oral first-in-class small molecule RSK inhibitor suppresses AR variants and tumor growth in prostate cancer [ Cancer Sci, 2022, 113(5):1731-1738] | PubMed: 35118769 |
| Impact of Androgen Receptor Activity on Prostate-Specific Membrane Antigen Expression in Prostate Cancer Cells [ Int J Mol Sci, 2022, 23(3)1046] | PubMed: 35162969 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
