|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| CAS No. | 946414-94-4 |
|---|---|
| Formulation | 100 mM Pro-Ac, 20 mM Arg, pH5.0 |
| Isotype | Human IgG4 |
| Source | CHO cells |
| Storage (From the date of receipt) |
Store the undiluted solution at 4°C in the dark to avoid freeze-thaw cycles |
| Purity | 99% |
| Protein concentration | 10.84mg/ml |
| Endotoxin Level | <1EU/mg |
Biological Activity
| Description | Nivolumab (anti-PD-1) is a genetically engineered, fully human immunoglobulin (Ig) G4 monoclonal antibody directed against the negative immunoregulatory human cell surface receptor programmed death-1 (PD-1,PCD-1) with immune checkpoint inhibitory and antineoplastic activities. MW : 143.597 KD. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | The cell binding ratio of 125I-Nivolumab and 125I-Nivolumab-DTPA to PD-1 is 13.78 ± 2.90% and 10.22 ± 2.62%, respectively. Conjugating of DTPA does not affect targeting of Nivolumab to PD-1 in vitro.[1] |
||||
| In vivo | In SPECT images, lesions with high 99mTc-DTPA-nivolumab uptake and relatively clear background are shown at 6 h. Thereafter, the suspected intestinal thickening in Gd-free T1WI is observed at 2 h after the addition of Gd-DTPA-nivolumab.[1] |
Protocol (Only for Reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
Customer Product Validation
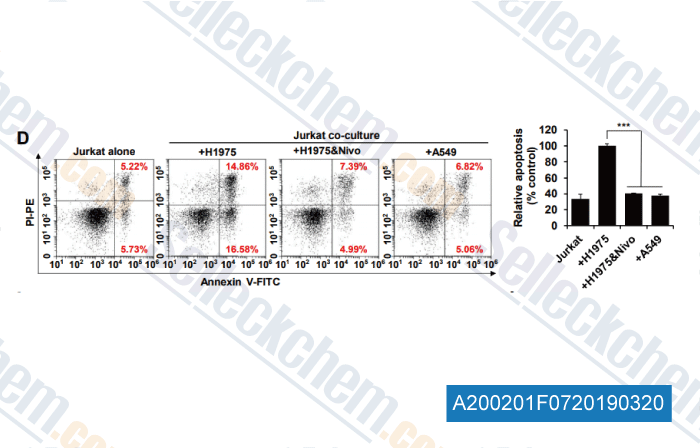
-
Data from [Data independently produced by , , Clin Cancer Res, 2019, doi:10.1158/1078-0432]
Apoptosis rates of Jurkat cells co-cultured for 72 h with H1975, A549 cells or H1975 with 10 μg/mL nivolumab were analyzed by Annexin V-FITC/PI assay (Left) and quantified (Right).
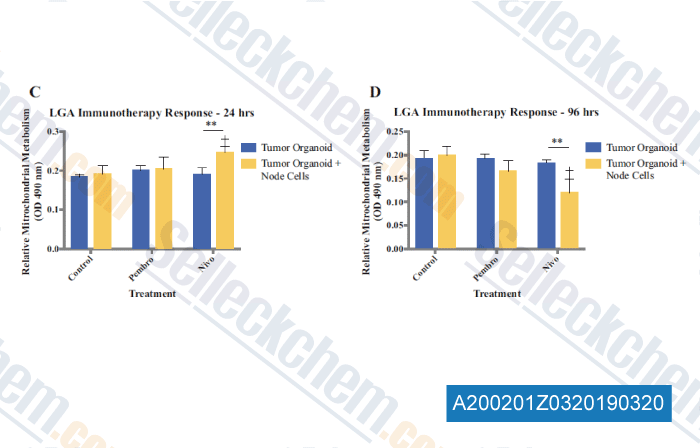
-
Data from [Data independently produced by , , Ann Surg Oncol, 2019, 26(1):139-147]
Preliminary immunotherapy drug screening using immune checkpoint inhibitors pembrolizumab and nivolumab in LGA organoids immune-enhanced with cells from a lymph node from the same patient. Mitochondrial metabolism was assessed at (c) 24 h and (d) 96 h after administration of the immunotherapy agents. Statistical significance: **p<0.05 between tumor cell-only and immune-enhanced organoids; p<0.05 between drug treatment and control of the same group.
Selleck's Nivolumab (anti-PD-1) has been cited by 87 publications
| NSUN2/ALYREF axis-driven m5C methylation enhances PD-L1 expression and facilitates immune evasion in non-small-cell lung cancer [ Cancer Immunol Immunother, 2025, 74(4):132] | PubMed: 40029463 |
| Therapeutic effect of fully human anti-Nrp-1 antibody on non-small cell lung cancer in vivo and in vitro [ Cancer Immunol Immunother, 2025, 74(2):50] | PubMed: 39751948 |
| Protocol for a patient-derived preclinical platform to model tumor-immune interactions and evaluate therapeutic efficacy [ STAR Protoc, 2025, 6(1):103623] | PubMed: 39918963 |
| Clinical drug screening reveals clofazimine potentiates the efficacy while reducing the toxicity of anti-PD-1 and CTLA-4 immunotherapy [ Cancer Cell, 2024, S1535-6108(24)00080-1] | PubMed: 38518774 |
| Base editing screens define the genetic landscape of cancer drug resistance mechanisms [ Nat Genet, 2024, 10.1038/s41588-024-01948-8] | PubMed: 39424923 |
| Human papillomavirus-encoded circular RNA circE7 promotes immune evasion in head and neck squamous cell carcinoma [ Nat Commun, 2024, 15(1):8609] | PubMed: 39366979 |
| Immunologic signatures of response and resistance to nivolumab with ipilimumab in advanced metastatic cancer [ J Exp Med, 2024, 221(10)e20240152] | PubMed: 39190534 |
| Modeling lung adenocarcinoma metastases using patient-derived organoids [ Cell Rep Med, 2024, 5(10):101777] | PubMed: 39413736 |
| Assessing personalized responses to anti-PD-1 treatment using patient-derived lung tumor-on-chip [ Cell Rep Med, 2024, S2666-3791(24)00241-6] | PubMed: 38703767 |
| Genomic and transcriptomic profiling of peripheral T cell lymphoma reveals distinct molecular and microenvironment subtypes [ Cell Rep Med, 2024, 5(2):101416] | PubMed: 38350451 |
FOR RESEARCH USE ONLY. NOT FOR USE IN HUMANS.
Specific storage and handling information for each product is indicated on the product datasheet. Most Selleck products are stable under the recommended conditions. Products are sometimes shipped at a temperature that differs from the recommended storage temperature. Short-term storage of many products are stable in the short-term at temperatures that differ from that required for long-term storage.
We ensure that the product is shipped under conditions that will maintain the quality of the reagents. Upon receipt of the product, follow the storage recommendations on the product data sheet.
