|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C23H34O5 |
|||
| Molecular Weight | 390.51 | CAS No. | 73573-88-3 | |
| Solubility (25°C)* | In vitro | DMSO | 78 mg/mL (199.73 mM) | |
| Ethanol | 7 mg/mL (17.92 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Mevastatin (ML-236B,Compactin) is a competitive inhibitor of HMG-Coenzyme A (HMG-CoA) reductase with a binding affinity 10,000 times greater than the HMG-CoA substrate itself. | |
|---|---|---|
| Targets |
|
|
| In vitro | Mevastatin is a cholesterol-lowering agent isolated from Penicillium citinium. It reduces cholesterol synthesis to 50% of control at 0.01 pg/mL (26 nM). [1] It is structurally similar to the HMG, a substituent of the endogenous substrate of HMG-CoA reductase. Mevastatin is a prodrug that is activated in vivo via hydrolysis of the lactone ring. The hydrolyzed lactone ring mimics the tetrahedral intermediate produced by the reductase allowing the agent to bind with 10,000 times greater affinity than its natural substrate. The bicyclic portion of mevastatin binds to the coenzyme A portion of the active site. Mevastatin increases levels of eNOS mRNA and protein, reduces infarct size, and improves neurological deficits in a dose- and time-dependent manner.[2] |
|
| In vivo | At doses of 5 and 20 mg/kg, mevastatin produces reduction of serum cholesterol levels at 3 hours after oral administration. It lowers the levels of serum cholesterol by approximately 30 % at a dose of 20 mg/kg. [1] Mevastatin lowers hepatic production of cholesterol by competitively inhibiting HMG-CoA reductase. Cholesterol levels are reduced only after 28 days of treatment and does not correlate with infarct reduction. Baseline absolute cerebral blood flow is 30% higher after 14-day high-dose treatment. [2] |
Protocol (from reference)
| Animal Study: |
|
|---|
References
|
Customer Product Validation

-
Data from [Data independently produced by , , Atherosclerosis, 2018, 276:28-38]
-
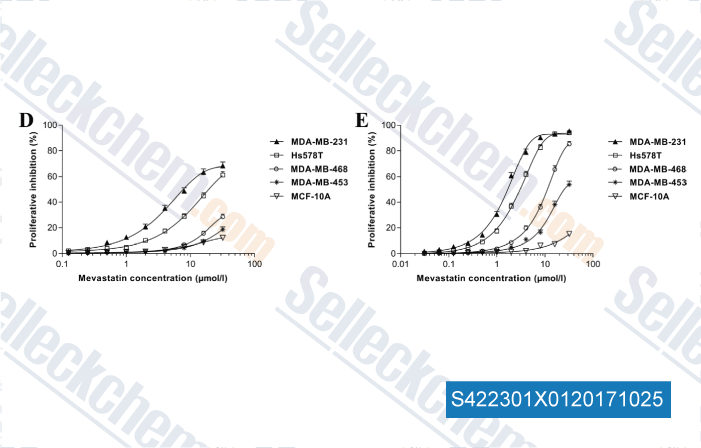
Data from [Data independently produced by , , Eur J Pharmacol, 2017, 813:161-171]
Selleck's Mevastatin has been cited by 9 publications
| Propafenone facilitates mitochondrial-associated ferroptosis and synergizes with immunotherapy in melanoma [ J Immunother Cancer, 2024, 12(11)e009805] | PubMed: 39581704 |
| Systems immunology-based drug repurposing framework to target inflammation in atherosclerosis [ Nat Cardiovasc Res, 2023, 2(6):550-571] | PubMed: 37771373 |
| The cholesterol metabolite 25-hydroxycholesterol restrains the transcriptional regulator SREBP2 and limits intestinal IgA plasma cell differentiation [ Immunity, 2021, 54(10):2273-2287.e6] | PubMed: 34644558 |
| Simvastatin Suppresses Human Breast Cancer Cell Invasion by Decreasing the Expression of Pituitary Tumor-Transforming Gene 1 [ Front Pharmacol, 2020, 11:574068] | PubMed: 33250768 |
| Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination [Hubler Z, et al. Nature, 2018, 560(7718):372-376] | PubMed: 30046109 |
| Simvastatin functions as a heat shock protein 90 inhibitor against triple-negative breast cancer [Kou X, et al. Cancer Sci, 2018, 109(10):3272-3284] | PubMed: 30039622 |
| MK-2206, an allosteric inhibitor of AKT, stimulates LDLR expression and LDL uptake: A potential hypocholesterolemic agent [Bjune K, et al. Atherosclerosis, 2018, 276:28-38] | PubMed: 30025252 |
| Triciribine increases LDLR expression and LDL uptake through stabilization of LDLR mRNA. [ Sci Rep, 2018, 8(1):16174] | PubMed: 30385871 |
| Vorinostat and Simvastatin have synergistic effects on triple-negative breast cancer cells via abrogating Rab7 prenylation. [Kou X, et al. Eur J Pharmacol, 2017, 813:161-171] | PubMed: 28826913 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
