|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C7H15N5O4.HCl |
|||
| Molecular Weight | 269.69 | CAS No. | 51298-62-5 | |
| Solubility (25°C)* | In vitro | Water | 54 mg/mL (200.22 mM) | |
| DMSO | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | L-NAME HCl (NG-Nitroarginine methyl ester, N-Nitro-L-arginine methylester) is a nonselective inhibitor of nitric oxide synthetases (NOS) for nNOS (bovine), eNOS (human), and iNOS (murine), with Ki of 15 nM, 39 nM and 4.4 μM, respectively. L-NAME HCl can be used to induce animal models of Hypertension. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | NG-nitro-L-arginine methyl ester (L-NAME; at 0.1-100 mM) causes concentration-dependent inhibition of the Ca2(+)-dependent endothelial NO synthase from porcine aortae. L-NAME causes an endothelium-dependent contraction and an inhibition of the endothelium-dependent relaxation induced by acetylcholine (ACh) in aortic rings. [2] In another research, Viability of rMC-1 cells or BREC in 25 mM glucose is significantly less than at 5 mM glucose, and this cell death is inhibited by l-NAME in both cell types. [3] | ||||
| In vivo | L-NAME (0.03-300 mg kg-1, i.v.) induces a dose-dependent increase in mean systemic arterial blood pressure accompanied by bradycardia. L-NAME (100 mg kg-1, i.v.) inhibits significantly the hypotensive responses to ACh and bradykinin. The increase in blood pressure and bradycardia produced by L-NAME is reversed by L-arginine (30-100 mg kg-1, i.v.) in a dose-dependent manner. [2] |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[3] |
|
| Animal Study:[2] |
|
References
Customer Product Validation
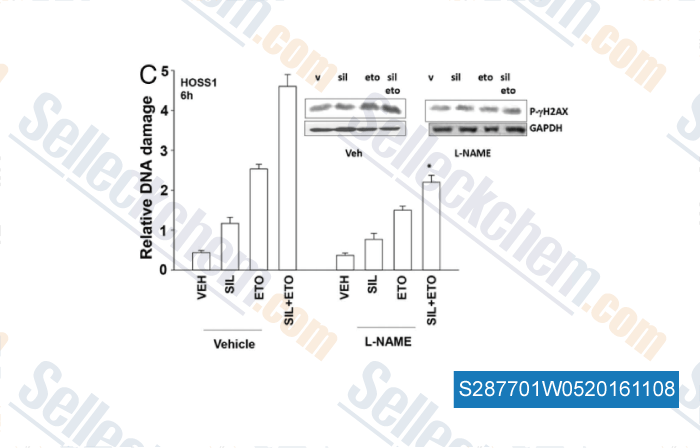
-
, , Cancer Biol Ther, 2014, 15(6):758-67.
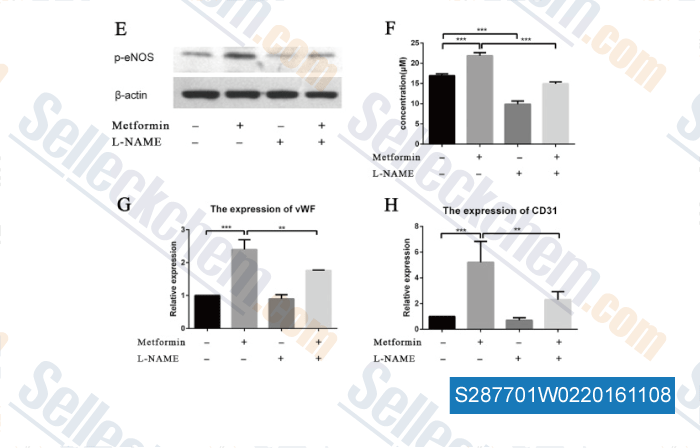
-
, , Biochem Biophys Res Commun, 2015, 465(4):803-9.
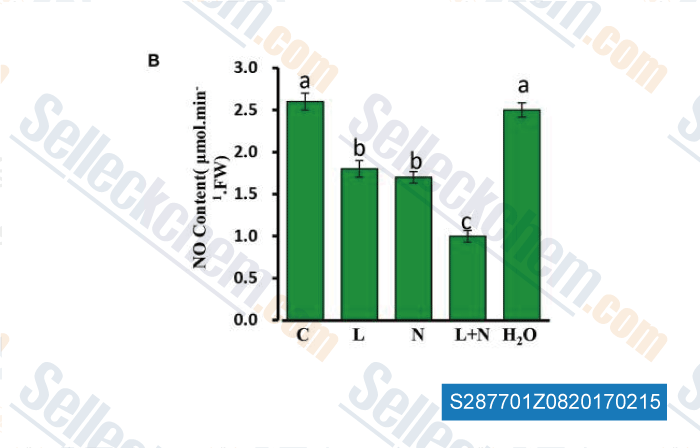
-
Data from [Data independently produced by , , Front Plant Sci, 2016, 7:1652.]
Selleck's L-NAME HCl has been cited by 42 publications
| Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation [ Nat Neurosci, 2024, 10.1038/s41593-024-01604-8] | PubMed: 38528202 |
| Nitric oxide facilitates the S-nitrosylation and deubiquitination of Notch1 protein to maintain cancer stem cells in human NSCLC [ J Cell Mol Med, 2024, 28(21):e70203] | PubMed: 39523215 |
| Mitochondrial Hyperactivity and Reactive Oxygen Species Drive Innate Immunity to the Yellow Fever Virus-17D Live-Attenuated Vaccine [ bioRxiv, 2024, 2024.09.04.611167] | PubMed: 39282299 |
| Ginsenoside Rg1 attenuates diabetic vascular endothelial dysfunction by inhibiting the calpain-1/ROS/PKC-β axis [ Life Sci, 2023, 329:121972] | PubMed: 37482213 |
| NMDA receptor activation induces damage of alveolar type II cells and lung fibrogenesis through ferroptosis [ Biochim Biophys Acta Mol Cell Res, 2023, 1870(7):119535] | PubMed: 37451346 |
| Critical role of mitogen-inducible gene 6 in restraining endothelial cell permeability to maintain vascular homeostasis [ J Cell Commun Signal, 2023, 17(1):151-165] | PubMed: 36284029 |
| Critical role of mitogen-inducible gene 6 in restraining endothelial cell permeability to maintain vascular homeostasis [ J Cell Commun Signal, 2023, 17(1):151-165] | PubMed: 36284029 |
| Ginsenoside Rg1 ameliorates chronic intermittent hypoxia-induced vascular endothelial dysfunction by suppressing the formation of mitochondrial reactive oxygen species through the calpain-1 pathway [ J Ginseng Res, 2023, 47(1):144-154] | PubMed: 36644390 |
| Vitamin C deficiency induces hypoglycemia and cognitive disorder through S-nitrosylation-mediated activation of glycogen synthase kinase 3β [ Redox Biol, 2022, 56:102420] | PubMed: 35969998 |
| Suppression of VEGFD expression by S-nitrosylation promotes the development of lung adenocarcinoma [ J Exp Clin Cancer Res, 2022, 41(1):239] | PubMed: 35941690 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
