|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C20H20N4O3 |
|||
| Molecular Weight | 364.4 | CAS No. | 888216-25-9 | |
| Solubility (25°C)* | In vitro | DMSO | 72 mg/mL (197.58 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Ganetespib (STA-9090) is an HSP90 inhibitor with IC50 of 4 nM in OSA 8 cells, induces apoptosis of OSA cells while normal osteoblasts are not affected; active metabolite of STA-1474. Phase 3. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | The 50% inhibitory concentrations (IC50) for Ganetespib against malignant mast cell lines are 10-50 times lower than that for 17-AAG, indicating that triazolone class of HSP90 inhibitors likely exhibits greater potency than geldanamycin based inhibitors. [1] Ganetespib inhibits MG63 cell lines with IC50 of 43 nM. [1] Ganetespib binds to the ATP-binding domain at the N-terminus of Hsp90 and serves as a potent Hsp90 inhibitor by causing degradation of multiple oncogenic Hsp90 client proteins including HER2/neu, mutated EGFR, Akt, c-Kit, IGF-1R, PDGFRα, Jak1, Jak2, STAT3, STAT5, HIF-1α, CDC2 and c-Met as well as Wilms' tumor 1. [2] Ganetespib, at low nanomolar concentrations, potently arrests cell proliferation and induces apoptosis in a wide variety of human cancer cell lines, including many receptor tyrosine kinase inhibitor- and tanespimycin-resistant cell lines. Ganetespib exhibits potent cytotoxicity in a range of solid and hematologic tumor cell lines, including those that express mutated kinases that confer resistance to small-molecule tyrosine kinase inhibitors. [3] Ganetespib treatment rapidly caused the degradation of known Hsp90 client proteins, exhibits superior potency to the ansamycin inhibitor 17-AAG, and shows sustained activity even with short exposure times.[3] In anohter study, Ganetespib induces apoptosis of malignant canine mast cell lines. Ganetespib is active at significantly lower concentrations for C2 and BR canine malignant mast cells with IC50 of 19 and 4 nM, respectively, while 17-AAG inhibits C2 and BR canine malignant mast cells with IC50 of 958 and 44 nM, respectively. [4] Both the expression of WT and mutant Kit are downregulated by 100 nM Ganetespib after 24 hours in all lines treated including C2 and BMCMCs cells. However, no effects on PI3K or HSP90 expression are observed following treatment with Ganetespib.[4] | ||
| In vivo | Administration of Ganetespib leads to significant tumor shrinkage in several tumor xenograft models in mice and appears to be less toxic. Furthermore Ganetespib demonstrated better tumor penetration compared with tanespimycin.[2] Ganetespib inhibits in vivo tumor growth in both malignant mast cell and OSA xenograft models. Ganetespib significantly inhibits tumor growth when dosed with two repeating cycles of 25 mg/kg/day for 3 days, with a %T/C value of 18. Ganetespib is well-tolerated, with the vehicle and Ganetespib groups having average bodyweight changes relative to the start of the study of +0.3% and -8.1% on day 17, respectively.[4] |
Protocol (from reference)
| Cell Assay:[1] |
|
|---|---|
| Animal Study:[4] |
|
References
Customer Product Validation
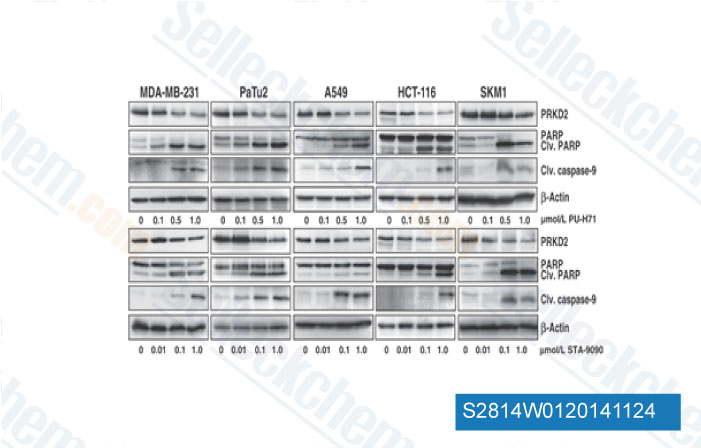
-
Data from [Data independently produced by Cancer Res, 2014, 10.1158/0008-5472.CAN-14-1017]
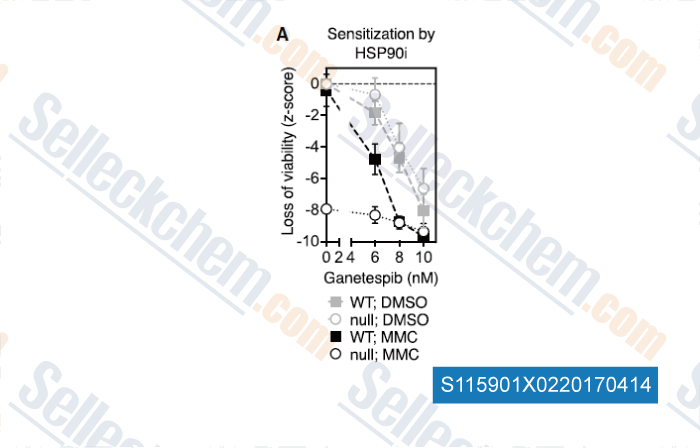
-
Data from [Data independently produced by , , Cell, 2017, 168(5):856-866]
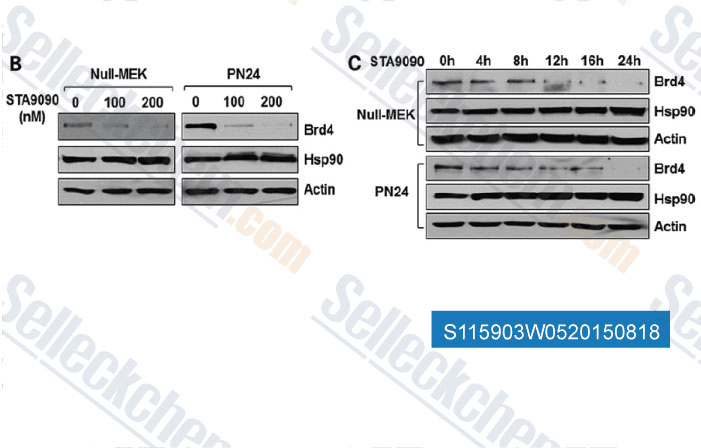
-
Data from [Data independently produced by , , Hum Mol Genet, 2015, 10.1093/hmg/ddv136]
Selleck's Ganetespib (STA-9090) has been cited by 78 publications
| HSC70 coordinates COP9 signalosome and SCF ubiquitin ligase activity to enable a prompt stress response [ EMBO Rep, 2025, 10.1038/s44319-025-00376-x] | PubMed: 39915298 |
| Impairment of lipid homeostasis causes lysosomal accumulation of endogenous protein aggregates through ESCRT disruption [ Elife, 2024, 12RP86194] | PubMed: 39713930 |
| Hsp90α promotes lipogenesis by stabilizing FASN and promoting FASN transcription via LXRα in hepatocellular carcinoma [ J Lipid Res, 2024, 66(1):100721] | PubMed: 39645039 |
| AFP-HSP90 mediated MYC/MET activation promotes tumor progression in hepatocellular carcinoma and gastric cancers [ Cancer Cell Int, 2024, 24(1):283] | PubMed: 39135041 |
| STA-9090 in combination with a statin exerts enhanced protective effects in rats fed a high-fat diet and exposed to diethylnitrosamine and thioacetamide [ Front Pharmacol, 2024, 15:1454829] | PubMed: 39309001 |
| Combined treatment with cetuximab and STA9090 has synergistic anticancer effects on human non-small cell lung cancer [ Acta Biochim Biophys Sin (Shanghai), 2024, 56(7):1022-1033] | PubMed: 38818581 |
| HSP90 inhibition suppresses tumor glycolytic flux to potentiate the therapeutic efficacy of radiotherapy for head and neck cancer [ Sci Adv, 2024, 10(8):eadk3663] | PubMed: 38394204 |
| The ALS-associated co-chaperone DNAJC7 mediates neuroprotection against proteotoxic stress by modulating HSF1 activity [ bioRxiv, 2024, 2024.12.01.626216] | PubMed: 39651147 |
| The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma [ Nat Commun, 2023, 14(1):1516] | PubMed: 36934113 |
| The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma [ Nat Commun, 2023, 14(1):1516] | PubMed: 36934113 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
