|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C43H53NO14.3H2O |
||||||||||
| Molecular Weight | 861.93 | CAS No. | 148408-66-6 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (116.01 mM) | ||||||||
| Ethanol | 100 mg/mL (116.01 mM) | ||||||||||
| Water | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Docetaxel (RP56976, NSC 628503), an analog of paclitaxel, is an inhibitor of depolymerisation of microtubules by binding to stabilized microtubules. | |
|---|---|---|
| Targets |
|
|
| In vitro | Docetaxel is a cytotoxic agent, especially for proliferating cells, which is related to its ability to promote the formation of microtubule bundles and induce sustained mitotic arrest, followed by apoptosis of mitotically arrested cells or permanent mitotic block. Docetaxel suppresses microtubule dynamic instability as well as tread-milling, resulting in the failure of chromosomes to segregate to the daughter cells, which in turn triggers premature exit from mitosis rather than a block at this phase of the cell cycle. [2] Docetaxel inhibits the clonogenic survival of Human cancer cell Hs746T (stomach), AGS (stomach), HeLa (cervix), CaSki (cervix), BxPC3 (pancreas), Capan-1 (pancreas) with IC50 of 1 nM, 1 nM, 0.3 nM, 0.3 nM, 0.3 nM and 0.3 nM respectively. [4] Docetaxel inhibits endothelial cell migration that does not affects microtubule gross morphology or inhibit cell proliferation, although they does produce more subtle effects on microtubule dynamics. Docetaxel inhibits HUVEC migration with an observed IC50 of 1 pM. HUVEC chemotaxis stimulated by either of two angiogenic factors, thymidine phosphorylase or VEGF, is inhibited by Docetaxel with IC 50 of 10 pM and is ablated at 1 nM. [7] Docetaxel induces human monocytes, but not RAW 264.7 murine macrophages, Prostaglandin H Synthase-2m (PGHS-2) expression. [8] | |
| In vivo | Docetaxel (33 mg/kg/dose, given i.v. every 4 days for 3 injections) results in a tumor growth delay of 19.3 days in M2OL2 colon xenografts. Docetaxel also shows great antitumor activities in MX-1, SK-MEL-2, LX-1 and OVCAR-3 xenografts. Docetaxel inhibits the angiogenic response to fibroblast growth factor 2 with IC 50 of 5.4 mg/kg when injected twice weekly over a 14-day period, and angiogenesis is completely blocked in mice that receives 10 mg/kg Docetaxel. Docetaxel has selectivity for endothelial cell migration and/or microvessel formation because infiltration of inflammatory cells into the Matrigel plug is much less sensitive to inhibition by Docetaxel. [7] |
Protocol (from reference)
| Cell Assay:[5] |
|
|---|---|
| Animal Study:[6] |
|
References
Customer Product Validation
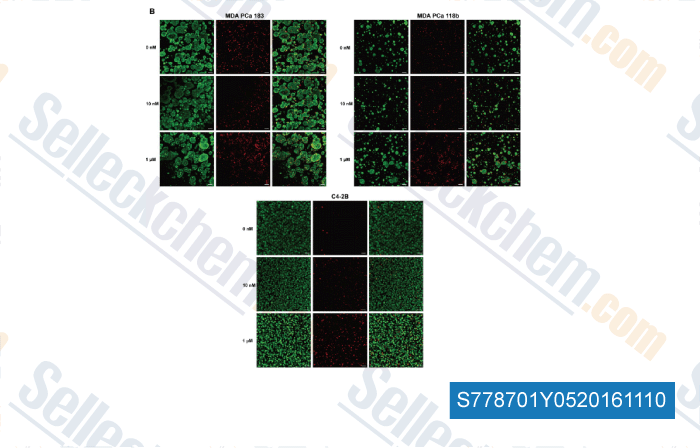
-
, , Mol Pharm, 2014, 11(7):2040-50.

-
, , J Transl Med, 2013, 11:204.
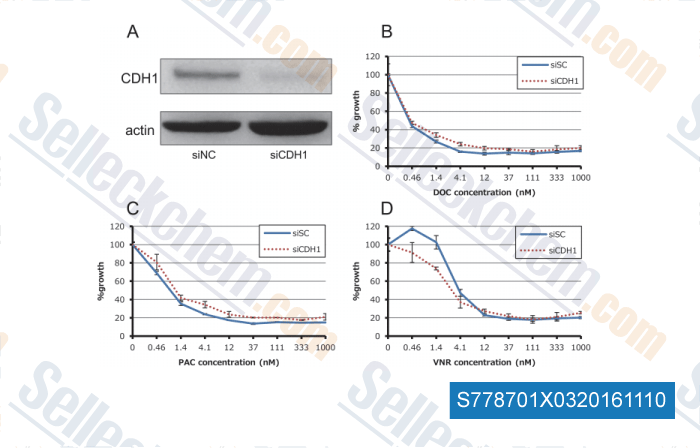
-
, , PLoS One,2015, 10(4):e0123901
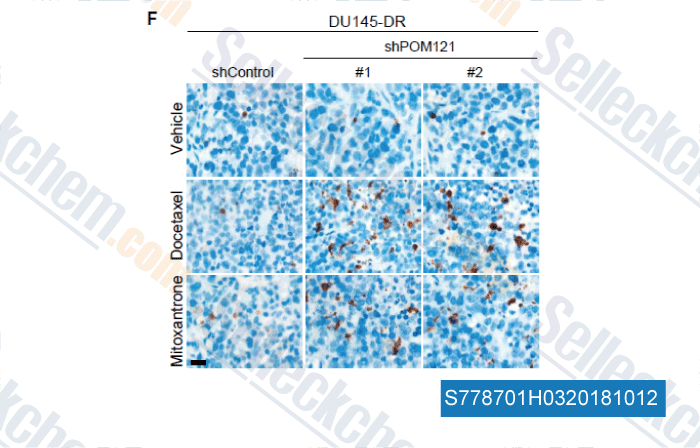
-
Data from [Data independently produced by , , Cell, 2018, 174(5):1200-1215]
Selleck's Docetaxel Trihydrate has been cited by 66 publications
| Combating castration-resistant prostate cancer by co-targeting the epigenetic regulators EZH2 and HDAC [ PLoS Biol, 2023, 21(4):e3002038] | PubMed: 37104245 |
| MARCKSL1-2 reverses docetaxel-resistance of lung adenocarcinoma cells by recruiting SUZ12 to suppress HDAC1 and elevate miR-200b [ Mol Cancer, 2022, 21(1):150] | PubMed: 35864549 |
| Docetaxel remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy [ Theranostics, 2022, 12(11):4965-4979] | PubMed: 35836810 |
| Therapeutic targeting of glutamate dehydrogenase 1 that links metabolic reprogramming and Snail-mediated epithelial-mesenchymal transition in drug-resistant lung cancer [ Pharmacol Res, 2022, 185:106490] | PubMed: 36216131 |
| The therapeutic effect of KSP inhibitors in preclinical models of cholangiocarcinoma [ Cell Death Dis, 2022, 13-9:799] | PubMed: 36123339 |
| Toll-Like Receptor 3 Overexpression Induces Invasion of Prostate Cancer Cells, whereas Its Activation Triggers Apoptosis [ Am J Pathol, 2022, S0002-9440(22)00174-2] | PubMed: 35750257 |
| Establishment and large-scale validation of a three-dimensional tumor model on an array chip for anticancer drug evaluation [ Front Pharmacol, 2022, 13:1032975] | PubMed: 36313330 |
| Imidazo[4,5-b]pyridine derived tubulin polymerization inhibitors: Design, synthesis, biological activity in vitro and computational analysis [ Bioorg Chem, 2022, 127:106032] | PubMed: 35872398 |
| Betacellulin promotes tumor development and EGFR mutant lung cancer growth by stimulating the EGFR pathway and suppressing apoptosis [ Bioengineered, 2022, 13(4):11310-11320] | PubMed: 35499128 |
| Gene therapy of prostate cancer using liposomes containing perforin expression vector driven by the promoter of prostate-specific antigen gene [ Sci Rep, 2022, 12(1):1442] | PubMed: 35087064 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
