|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C27H29NO10 . HCl |
||||||
| Molecular Weight | 563.98 | CAS No. | 23541-50-6 | ||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (177.31 mM) | ||||
| Water | 25 mg/mL (44.32 mM) | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Daunorubicin HCl inhibits both DNA and RNA synthesis and inhibits DNA synthesis with Ki of 0.02 μM in a cell-free assay. Daunorubicin is a topoisomerase II inhibitor that induces apoptosis.Daunorubicin (RP 13057) HCl can be used to induce animal models of Kidney Disease. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | At drug concentrations that reflect the peak plasma concentration after Daunorubicin administration, the primary mechanism is likely to be through interaction with topoisomerase II, which may be a primary triggering event for growth arrest and/or cell killing through a signalling pathway leading to apoptosis, at least in leukemic cells and thymocytes. The quinone structure permits daunorubicin to act as electron acceptors in reactions mediated by oxoreductive enzymes including cytochrome P450 reductase, NADH dehydrogenase, and xanthine oxidase. At Daunorubicin concentrations exceeding approximately 2–4 μM, free radical mediated toxicity and DNA cross-linking may become evident. Daunorubicin inhibits both DNA and RNA syntheses in HeLa cells over a concentration range of 0.2 through 2 μM. Daunorubicin inhibits both DNA syntheses in Ehrlich ascites tumor cells over a concentration range of 4 μM. Daunorubic triggers apoptosis at concentrations of 0.5 and 1 μM in either HL-60 or U-937 human leukemic cells. [1] Daunorubicin stimulates ceramide elevation and apoptosis in P388 and U937 cells through de novo synthesis via activation of the enzyme ceramide synthase. [2] Daunorubicin dose-dependently increases the phosphatidylserine exposure and consequent procoagulant activity of human umbilical vein endothelial cells. Daunorubicin (0.2 mM) significantly enhances the release of endothelial microparticles which are highly procoagulant in human umbilical vein endothelial cells. [3] |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
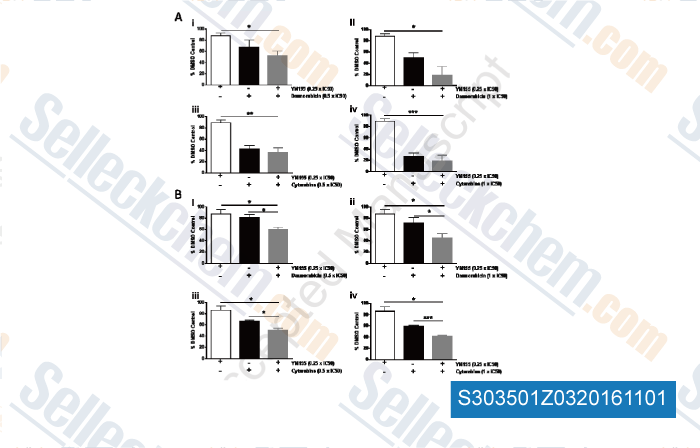
-
, , Leuk Res, 2015, 39(4):435-44.
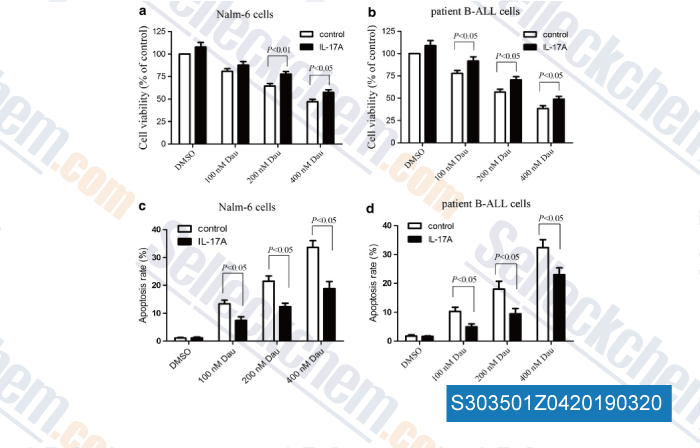
-
Data from [Data independently produced by , , J Transl Med, 2016, 14(1):132]
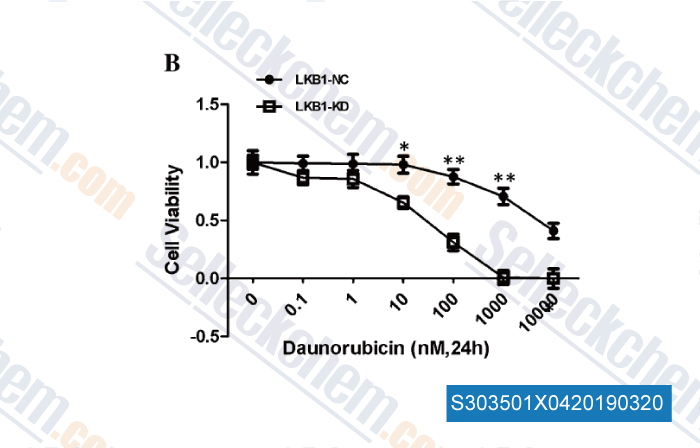
-
Data from [Data independently produced by , , Mol Cell Biochem, 2017, 434(1-2):25-32]
Selleck's Daunorubicin HCl has been cited by 80 publications
| Oncogenic role of RARG rearrangements in acute myeloid leukemia resembling acute promyelocytic leukemia [ Nat Commun, 2025, 16(1):617] | PubMed: 39805831 |
| Orthogonal proteogenomic analysis identifies the druggable PA2G4-MYC axis in 3q26 AML [ Nat Commun, 2024, 15(1):4739] | PubMed: 38834613 |
| CASZ1 upregulates PI3K-AKT-mTOR signaling and promotes T-cell acute lymphoblastic leukemia [ Haematologica, 2024, 109(6):1713-1725] | PubMed: 38058200 |
| Daunorubicin induces GLI1‑dependent apoptosis in colorectal cancer cell lines [ Int J Oncol, 2024, 64(6)66] | PubMed: 38757343 |
| Chemotherapy-initiated cysteine-rich protein 61 decreases acute B-lymphoblastic leukemia chemosensitivity [ J Cancer Res Clin Oncol, 2024, 150(3):159] | PubMed: 38530432 |
| Immuno-oncological effects of standard anticancer agents and commonly used concomitant drugs: an in vitro assessment [ BMC Pharmacol Toxicol, 2024, 25(1):25] | PubMed: 38444002 |
| Immuno-oncological effects of standard anticancer agents and commonly used concomitant drugs: an in vitro assessment [ BMC Pharmacol Toxicol, 2024, 25(1):25] | PubMed: 38444002 |
| SRCAP mutations drive clonal hematopoiesis through epigenetic and DNA repair dysregulation [ Cell Stem Cell, 2023, 10.1016/j.stem.2023.09.011] | PubMed: 37863054 |
| Transcriptional Response to Standard AML Drugs Identifies Synergistic Combinations [ Int J Mol Sci, 2023, 24(16)12926] | PubMed: 37629110 |
| Transcriptional Response to Standard AML Drugs Identifies Synergistic Combinations [ Int J Mol Sci, 2023, 24(16)12926] | PubMed: 37629110 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
