|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| CAS No. | 205923-56-4 |
|---|---|
| Formulation | 100 mM Pro-Ac, 20 mM Arg, pH5.0 |
| Isotype | Human IgG1 |
| Source | CHO cells |
| Storage (From the date of receipt) |
Store the undiluted solution at 4°C in the dark to avoid freeze-thaw cycles |
| Purity | 99% |
| Protein concentration | 8.1mg/ml |
| Endotoxin Level | <1EU/mg |
Biological Activity
| Description | Cetuximab, a novel molecular-targeted agent,is an inhibitor of EGFR monoclonal humanized antibody interacting with the extracellular binding site of EGFR to block ligand stimulation. MW:145.781 KD. |
|---|---|
| In vitro | Cetuximab treatment increases mitochondrial priming of EGFR-expressing HeLa cells but not in EGFR-expressing MDA-MD-231 cells.[2] |
| In vivo | C225 enhanced the antitumor activity of several chemotherapeutic drugs in mouse xenograft models[1]. Cetuximab, exerts its antitumor efficacy by multiple mechanisms that include the inhibition of cell cycle progression by arrest in the G1- phase and decreased cell number in the S-phase. Cell cycle arrest in the G1-phase also induces apoptosis by the induction and activation of proapoptotic molecules. cetuximab alone and in synergy with carboplatin resulted in decreases of tumor size, metastatic spread, and MVD in NCI-N87 tumors with EGFR cell surface expression and absence of mutations in BRAF and K-ras, whereas cetuximab had minimal in vitro effect and no in vivo treatment efficacy in tumors derived from MKN-45, in which the phenotype was also BRAF and K-ras wildtype, but which had only weak cytoplasmic EGFR protein expression[3]. |
Protocol (Only for Reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
| References |
|
|---|
Customer Product Validation
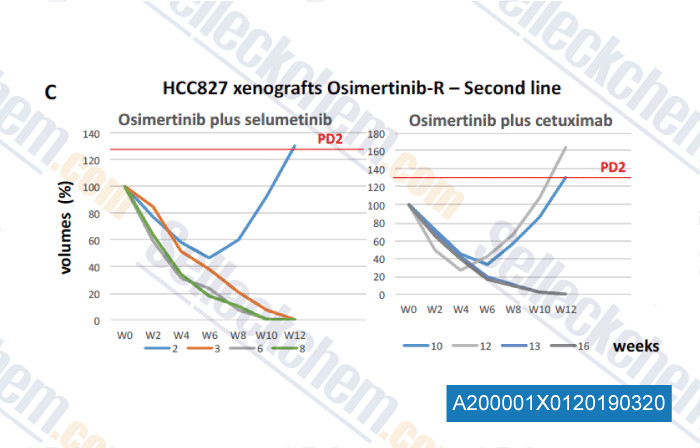
-
Data from [Data independently produced by , , J Thorac Oncol, 2018, 13(6):810-820]
Mice showed progression disease to first-line treatment were randomized 1:1 to the two arms of treatment and were treated until onset of progression disease or until the end-time of experiment in case of response (fixed to 52 weeks from the start of first-line therapy ), as indicated in in the Materials and Methods section. Growth curves of HCC827 xenografts treated in secondline with osimertinib plus selumetinib or cetuximab are represented as changes in the values of volumes as percentage compared to baseline tumor volume at the time of progression diasease to first-line (defined as 100%) for each case. Median tumor volume at PD2 was of 348 mm3.
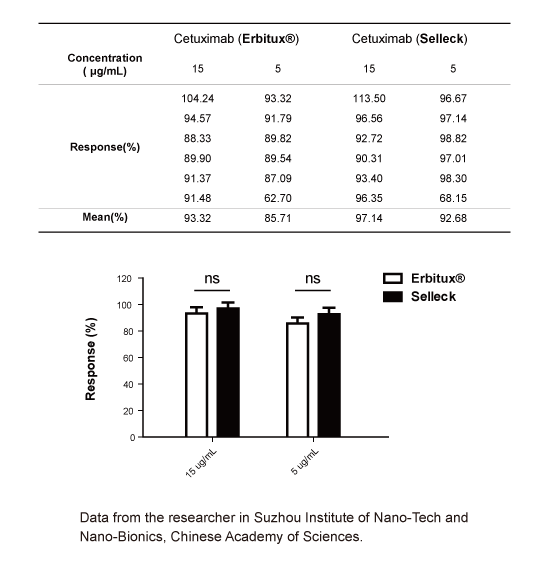
-
Data from [Data independently produced by , , ]
Selleck's Cetuximab (anti-EGFR) has been cited by 52 publications
| Oncogenic RARγ isoforms promote head and neck cancer proliferation through vinexin-β-mediated cell cycle acceleration and autocrine activation of EGFR signal [ Int J Biol Sci, 2025, 21(1):1-16] | PubMed: 39744424 |
| Base editing screens define the genetic landscape of cancer drug resistance mechanisms [ Nat Genet, 2024, 10.1038/s41588-024-01948-8] | PubMed: 39424923 |
| The effector program of human CD8 T cells supports tissue remodeling [ J Exp Med, 2024, 221(2)e20230488] | PubMed: 38226976 |
| EGFR-directed antibodies promote HER2 ADC internalization and efficacy [ Cell Rep Med, 2024, S2666-3791(24)00539-1] | PubMed: 39437778 |
| EGFR and HER2 hyper-activation mediates resistance to endocrine therapy and CDK4/6 inhibitors in ER+ breast cancer [ Cancer Lett, 2024, 593:216968] | PubMed: 38788968 |
| Label-free mapping of cetuximab in multi-layered tumor oral mucosa models by atomic force-microscopy-based infrared spectroscopy [ Analyst, 2024, 149(7):2122-2130] | PubMed: 38436119 |
| Label-free mapping of cetuximab in multi-layered tumor oral mucosa models by atomic force-microscopy-based infrared spectroscopy [ Analyst, 2024, 149(7):2122-2130] | PubMed: 38436119 |
| CDK4/6 inhibition to resensitize BRAF/EGFR inhibitor in patient-derived BRAF/PTEN-mutant colon cancer cells [ Transl Cancer Res, 2024, 13(7):3695-3703] | PubMed: 39145064 |
| Differential Regulation of the STING Pathway in Human Papillomavirus-Positive and -Negative Head and Neck Cancers [ Cancer Res Commun, 2024, 4(1):118-133] | PubMed: 38147007 |
| Double Digital Assay for Single Extracellular Vesicle and Single Molecule Detection [ Adv Sci (Weinh), 2023, 10(33):e2303619] | PubMed: 37802976 |
FOR RESEARCH USE ONLY. NOT FOR USE IN HUMANS.
Specific storage and handling information for each product is indicated on the product datasheet. Most Selleck products are stable under the recommended conditions. Products are sometimes shipped at a temperature that differs from the recommended storage temperature. Short-term storage of many products are stable in the short-term at temperatures that differ from that required for long-term storage.
We ensure that the product is shipped under conditions that will maintain the quality of the reagents. Upon receipt of the product, follow the storage recommendations on the product data sheet.
