|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C16H24O4 |
|||
| Molecular Weight | 280.36 | CAS No. | 20350-15-6 | |
| Solubility (25°C)* | In vitro | DMSO | 125 mg/mL warmed with 50ºC water bath (445.85 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Brefeldin A (BFA) is a lactone antibiotic and ATPase inhibitor for protein transport with IC50 of 0.2 μM in HCT 116 cells, induces cancer cell differentiation and apoptosis. It could also improve the HDR(homology-directed repair) efficiency and be an enhancer of CRISPR-mediated HDR. Brefeldin A is also an inhibitor of autophagy and mitophagy. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Brefeldin A is a fungal metabolite and blocks the forward transport between the endoplasmic reticulum and Golgi apparatus, Brefeldin A causes an impaired distribution of the membrane proteins. When HCT 116 human colon cancer cell is treated with Brefeldin A, morphological changes indicating cell differentiation are observed. Brefeldin A exerts its cytotoxic effects mainly by inducing differentiation and apoptosis in tumor cells. [1] The treatment of the strips with 20 μg/mL Brefeldin A for 6 hours completely abolishes the relaxation induced by bradykinin in the presence of 10mM indomethacin and 30 μM L-NOARG. The treatment with 20 μg/mL Brefeldin A substantially abolishes the bradykinin-induced decreases in [Ca2+]i and tension in the range of concentrations between 1 nM and 1 mM. Brefeldin A has no effect on the [Ca2+]i elevation in endothelial cells induced by bradykinin or substance P. [2] Addition of the fungal metabolite Brefeldin A does not affect the spontaneous phospholipid-dependent GTPS binding to myr-rARF1 but totally abolishs the retinal isotonic extract (RIE)-catalyzed exchange, with half-maximal inhibition at 2 μM Brefeldin A. Brefeldin A prevents a wide variety of membrane traffic pathways. Brefeldin A inhibits an ADP-ribosylation factor-specific guanine nucleotide exchange activity present in Golgi membranes or in brain cytosol. The complete prevention by Brefeldin A strongly suggests that the retinal extract contains an ARF-specific guanine nucleotide exchange factor. Retinal isotonic extract (RIE)-catalyzed GTPS release from both ADP-ribosylation factors (ARFs) is only partly inhibited by Brefeldin A, even at 300 μM. [3] Brefeldin A induces fusion of the Golgi apparatus with the ER. Brefeldin A abolishes the inhibitory effect of the CERT inhibitor HPA-12. Brefeldin A treatment, which induces fusion of the Golgi apparatus and the ER, rescues the limonoid-induced prevention of sphingomyelin biosynthesis. BFA treatment of CHO cells causes a 2 to 3 fold increase in sphingomyelin synthesis. [4] Apart from B-CLL cells, Brefeldin A reportedly causes apoptosis in multiple myeloma (U266, NCI-H929), Jurkat, HeLa, leukaemia (HL60, K562, BJAB), colon (HT-29) and prostate, as well as adenoid cystic sarcoma cells. The administration of 25 ng/mL of Brefeldin A completely blocks growth of HF4.9 and HF28RA cells, whereas higher Brefeldin A doses (75 ng/mL) are required to achieve the same effect in HF1A3 cells. Cell proliferation is inhibited within 24 hours in a dose-dependent manner and, depending on the cell line, almost complete cessation of 3H-thymdine incorporation is observed at 50-75 ng/mL of Brefeldin A (26%, 76%, 87% inhibition at 50 ng/ml and 75%, 87%, 92% inhibition at 75 ng/mL for HF1A3, HF4.9 and HF28RA cells respectively. Brefeldin A-induced cell killing is in a dose-dependent manner using YO-PRO 1/PI assay. [5] Brefeldin A could improve the HDR(homology-directed repair) efficiency. It is an enhancer of CRISPR-mediated HDR[6]. |
||
| In vivo | Brefeldin A (BFA) is a lactone antibiotic and a specific inhibitor of protein trafficking. Brefeldin A blocks the transport of secreted and membrane proteins from endoplasmic reticulum to Golgi apparatus. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
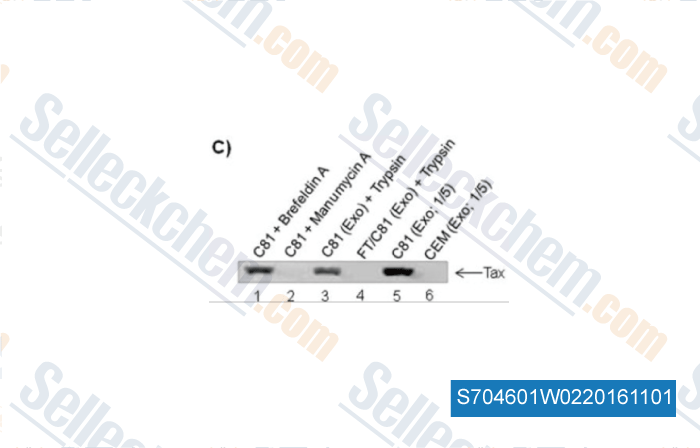
-
, , J Biol Chem, 2014, 289(32):22284-305.
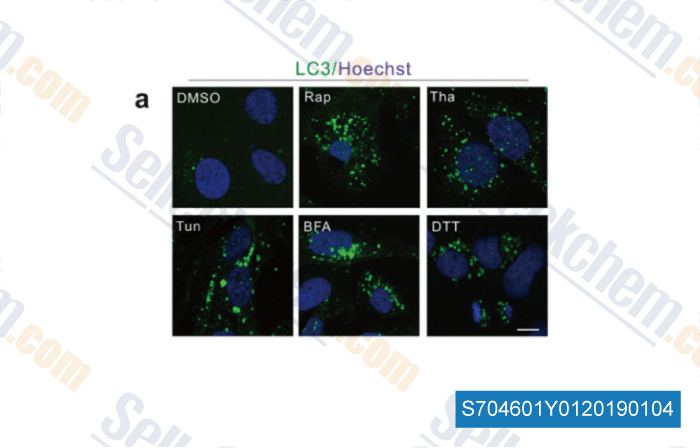
-
Data from [Data independently produced by , , Nanoscale, 2018, 10(18):8796-8805]
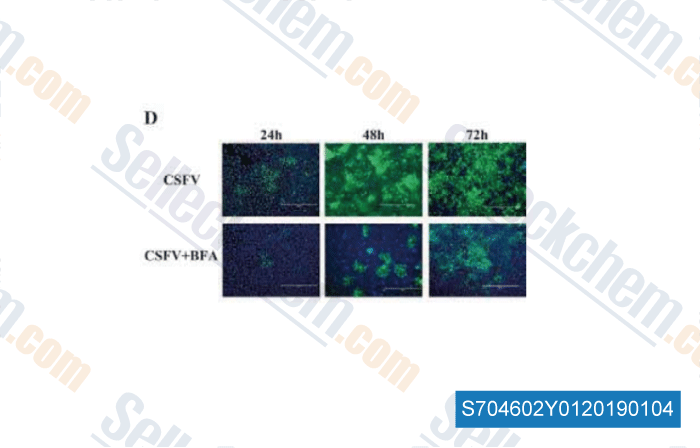
-
Data from [Data independently produced by , , J Biosci, 2018, 42(1):43-56]
Selleck's Brefeldin A (BFA) has been cited by 86 publications
| Neutrophil extracellular traps license macrophage production of chemokines to facilitate CD8+ T cell infiltration in obstruction-induced renal fibrosis [ Protein Cell, 2025, pwaf020] | PubMed: 39998389 |
| Targeting IL-17A enhances imatinib efficacy in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia [ Nat Commun, 2024, 15(1):203] | PubMed: 38172124 |
| Leptin-mediated suppression of lipoprotein lipase cleavage enhances lipid uptake and facilitates lymph node metastasis in gastric cancer [ Cancer Commun (Lond), 2024, 10.1002/cac2.12583] | PubMed: 38958445 |
| A dual-STING-activating nanosystem expands cancer immunotherapeutic temporal window [ Cell Rep Med, 2024, S2666-3791(24)00544-5] | PubMed: 39454571 |
| FOXA1 enhances antitumor immunity via repressing interferon-induced PD-L1 expression in nasopharyngeal carcinoma [ J Immunother Cancer, 2024, 12(11)e010091] | PubMed: 39542656 |
| ISGylation by HERCs facilitates STING activation [ Cell Rep, 2024, 43(5):114135] | PubMed: 38652662 |
| IRE1 RNase controls CD95-mediated cell death [ EMBO Rep, 2024, 25(4):1792-1813] | PubMed: 38383861 |
| CircGSK3β mediates PD-L1 transcription through miR-338-3p/PRMT5/H3K4me3 to promote breast cancer cell immune evasion and tumor progression [ Cell Death Discov, 2024, 10(1):426] | PubMed: 39366935 |
| H5 subtype avian influenza virus induces Golgi apparatus stress response via TFE3 pathway to promote virus replication [ PLoS Pathog, 2024, 20(12):e1012748] | PubMed: 39652582 |
| Caspase-1 in Cx3cr1-expressing cells drives an IL-18-dependent T cell response that promotes parasite control during acute Toxoplasma gondii infection [ PLoS Pathog, 2024, 20(10):e1012006] | PubMed: 39446964 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
