|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C30H34N2O3.HCl |
||||||||||||||
| Molecular Weight | 507.06 | CAS No. | 198480-56-7 | ||||||||||||
| Solubility (25°C)* | In vitro | DMSO | 90 mg/mL (177.49 mM) | ||||||||||||
| Water | Insoluble | ||||||||||||||
| Ethanol | Insoluble | ||||||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Bazedoxifene HCl (WAY-140424, TSE-424) is a novel, non-steroidal, indole-based estrogen receptor modulator (SERM) binding to both ERα and ERβ with IC50 of 23 nM and 89 nM, respectively. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Bazedoxifene is a third generation selective estrogen receptor modulator (SERM). Bazedoxifene does not stimulate ERα mediated transcriptional activity and acts as an antagonist to estradiol in cultured breast cancer (bMCF-7) cells. Similar results are seen in other cell lines including CHO (ovarian), HepG2 (hepatic) or GTI-7 (neuronal) with bazedoxifene having no ERα agonist activity and acting as an antagonist to estradiol action.[2] Bazedoxifene does not stimulate proliferation of MCF-7 cells but did inhibit 17β-estradiol-induced proliferation with IC50 of 0.19 nM. [3] | ||||
| In vivo | In an immature rat model, bazedoxifene increases uterine wet weight 35% at a dose of 0.5 mg/kg compared to an 85% increase with raloxifene at the same dose and a 300% increase in uterine weight with ethinyl estradiol at a dose of 10 μg/kg. Ovarectomized rats treated with 0.3 mg/d bazedoxifene displayed maintenance of bone mass and bone strength similar to effects seen with 2 μg/d ethinyl estradiol, 3 mg/d raloxifene, or sham operated animals. [2] |
Protocol (from reference)
| Kinase Assay:[3] |
|
|---|---|
| Cell Assay:[3] |
|
| Animal Study:[2] |
|
References
|
Customer Product Validation
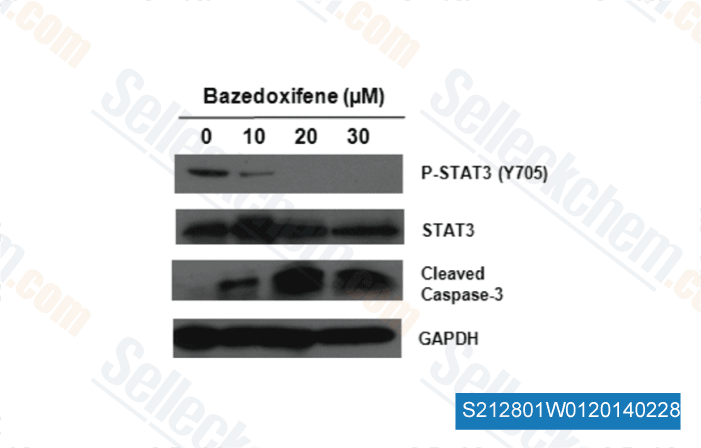
-
Data from [ J Med Chem , 2014 , 57(3), 632-41 ]
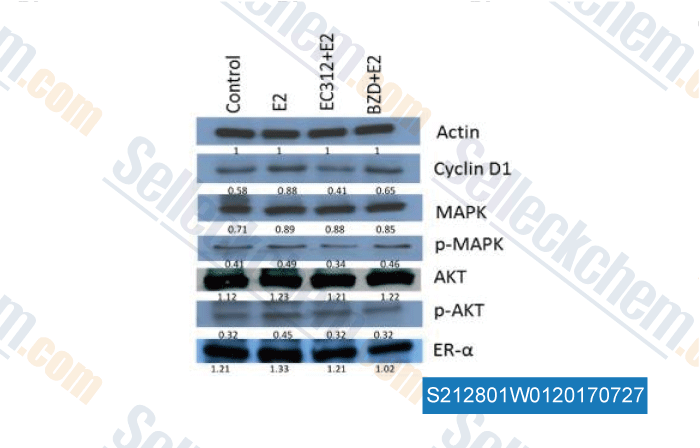
-
Data from [ , , PLoS One, 2016, 24;11(3):e0151182 ]
Selleck's Bazedoxifene (WAY-140424) HCl Has Been Cited by 18 Publications
| Combination of bazedoxifene with chemotherapy and SMAC-mimetics for the treatment of colorectal cancer [ Cell Death Dis, 2024, 15(4):255] | PubMed: 38600086 |
| Patient-derived rhabdomyosarcoma cells recapitulate the genetic and transcriptomic landscapes of primary tumors [ iScience, 2024, 27(10):110862] | PubMed: 39319271 |
| The Interleukin-11/IL-11 Receptor Promotes Glioblastoma Survival and Invasion under Glucose-Starved Conditions through Enhanced Glutaminolysis [ Int J Mol Sci, 2023, 24(4)3356] | PubMed: 36834778 |
| Targeting the gp130/STAT3 Axis Attenuates Tumor Microenvironment Mediated Chemoresistance in Group 3 Medulloblastoma Cells [ Cells, 2022, 11(3)381] | PubMed: 35159191 |
| Utilizing an Endogenous Progesterone Receptor Reporter Gene for Drug Screening and Mechanistic Study in Endometrial Cancer [ Cancers (Basel), 2022, 14(19)4883] | PubMed: 36230806 |
| A compendium of kinetic modulatory profiles identifies ferroptosis regulators [ Nat Chem Biol, 2021, 10.1038/s41589-021-00751-4] | PubMed: 33686292 |
| Synergistic PIM kinase and proteasome inhibition as a therapeutic strategy for MYC-overexpressing triple-negative breast cancer [ Cell Chem Biol, 2021, S2451-9456(21)00400-1] | PubMed: 34525344 |
| Bazedoxifene inhibits sustained STAT3 activation and increases survival in GBM [ Transl Oncol, 2021, 14(11):101192] | PubMed: 34365219 |
| Autocrine and paracrine signaling contributes to acquired chemotherapeutic resistance in Group 3 medulloblastoma [ B.S., The University of Arizona, 2021, ] | PubMed: none |
| The dysregulated pharmacology of clinically relevant ESR1 mutants is normalized by ligand-activated WT receptor. [ Mol Cancer Ther, 2020, 7 pii: molcanther] | PubMed: 32381587 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
