|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C32H43NO4 |
|||
| Molecular Weight | 505.69 | CAS No. | 218600-53-4 | |
| Solubility (25°C)* | In vitro | DMSO | 21 mg/mL (41.52 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Bardoxolone Methyl (RTA 402, TP-155, NSC 713200, CDDO Methyl Ester, CDDO-Me) is an IKK inhibitor, showing potent proapoptotic and anti-inflammatory activities; Also a potent Nrf2 activator and nuclear factor-κB (NF-κB) inhibitor. Bardoxolone Methyl abrogates ferroptosis. Bardoxolone methyl induces apoptosis and autophagy in cancer cells. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Bardoxolone Methyl exhibits potent inhibitory activities against production of nitric oxide induced by interferon-Ƴ in mouse macrophages with IC50 of 0.1 nM. [1] Bardoxolone Methyl decreases the viability of leukemic HL-60, KG-1, and NB4 cells with IC50 of 0.4, 0.4, and 0.27 μM, respectively. CDDO-Me induces pro-apoptotic Bax protein, inhibits the activation of ERK1/2, and it blocks Bcl-2 phosphorylation, which contributes to the induction of apoptosis. [2] Bardoxolone Methyl potently inhibits both constitutive and inducible NF-kappaB activated by TNF, interleukin (IL)-1beta, phorbol ester, okadaic acid, hydrogen peroxide, lipopolysaccharide, and cigarette smoke. [3] |
||||
| In vivo | Bardoxolone Methyl (60 mg/kg) reduces the number, size, and severity of lung tumors in vivo. [4] Bardoxolone Methyl significantly reduces the in vivo inflammatory cytokine response following LPS challenge, induces HO-1 protein expression in the spleen, and protects mice against lethal-dose LPS. [5] |
||||
| Features | The only IKKβ inhibitor in clinical use for solid tumors, type 2 diabetes, and chronic kidney disease. An orally-available antioxidant inflammation modulator. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
Customer Product Validation
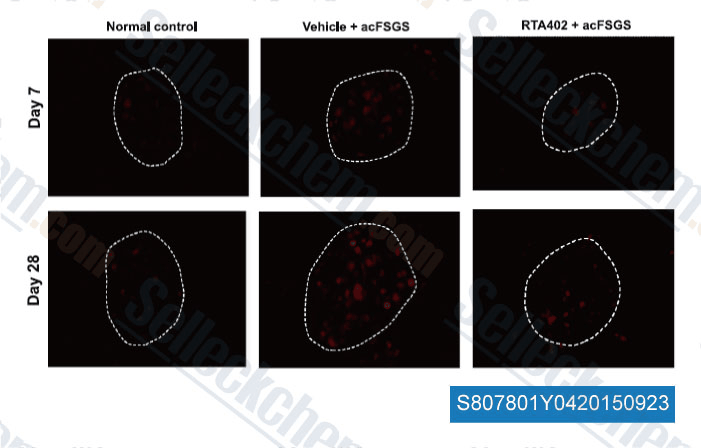
-
Data from [Data independently produced by , , Free Radic Biol Med, 2014, 73:260-9 ]
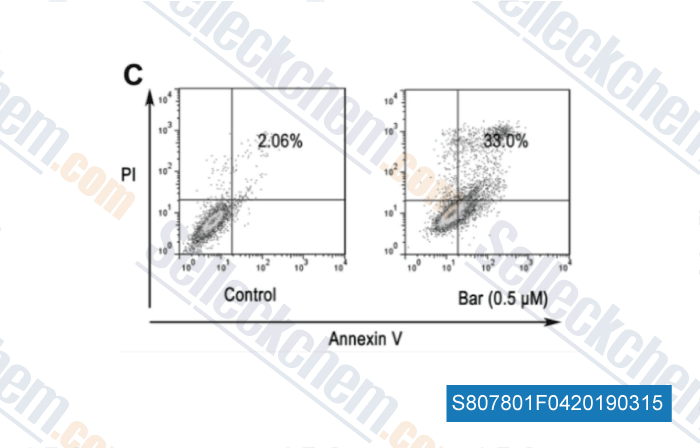
-
Data from [Data independently produced by , , Oncol Rep, 2017, 38(3):1517-1524]
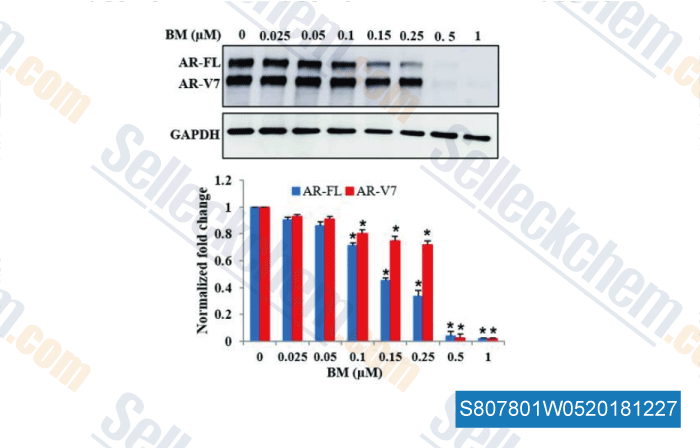
-
Data from [Data independently produced by , , Oncol Rep, 2017, 38(5):2774-2786]
Selleck's Bardoxolone Methyl has been cited by 32 publications
| Macrophage ILF3 promotes abdominal aortic aneurysm by inducing inflammatory imbalance in male mice [ Nat Commun, 2024, 15(1):7249] | PubMed: 39179537 |
| Establishment of human pluripotent stem cell-derived cortical neurosphere model to study pathomechanisms and chemical toxicity in Kleefstra syndrome [ Sci Rep, 2024, 14(1):22572] | PubMed: 39343771 |
| USP25 regulates KEAP1-NRF2 anti-oxidation axis and its inactivation protects acetaminophen-induced liver injury in male mice [ Nat Commun, 2023, 14(1):3648] | PubMed: 37339955 |
| Association of NRF2 with HIF-2α-induced cancer stem cell phenotypes in chronic hypoxic condition [ Redox Biol, 2023, 60:102632] | PubMed: 36791645 |
| Human iPSC-derived renal collecting duct organoid model cystogenesis in ADPKD [ Cell Rep, 2023, 42(12):113431] | PubMed: 38039961 |
| Transcriptional landscape of mitochondrial electron transport chain inhibition in renal cells [ Cell Biol Toxicol, 2023, 10.1007/s10565-023-09816-7] | PubMed: 37353587 |
| Synthetic oleanane triterpenoids suppress MYB oncogene activity and sensitize T-cell acute lymphoblastic leukemia cells to chemotherapy [ Front Oncol, 2023, 13:1126354] | PubMed: 37077825 |
| 2,3,5,4'-Tetrahydroxystilbene -TG1), a Novel Compound Derived from 2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside -THSG), Inhibits Colorectal Cancer Progression by Inducing Ferroptosis, Apoptosis, and Autophagy [ Biomedicines, 2023, 11(7)1798] | PubMed: 37509438 |
| Snail acetylation by autophagy-derived acetyl-coenzyme A promotes invasion and metastasis of KRAS-LKB1 co-mutated lung cancer cells [ Cancer Commun (Lond), 2022, 42(8):716-749] | PubMed: 35838183 |
| Snail acetylation by autophagy-derived acetyl-coenzyme A promotes invasion and metastasis of KRAS-LKB1 co-mutated lung cancer cells [ Cancer Commun (Lond), 2022, 10.1002/cac2.12332] | PubMed: 35838183 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
