|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C25H20N4O5 |
||||||||||
| Molecular Weight | 456.45 | CAS No. | 147403-03-0 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 91 mg/mL (199.36 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Azilsartan (TAK-536) is an angiotensin II type 1 (AT1) receptor antagonist with IC50 of 2.6 nM. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Azilsartan inhibits the specific binding of 125I-Sar1-Ile8-AII to human angiotensin type 1 receptors. This compound also inhibits the accumulation of AII-induced inositol 1-phosphate (IP1) in the cell-based assay with an IC50 value of 9.2 nM. In isolated rabbit aortic strips, it reduces the maximal contractile response to AII with a pD'2 value of 9.9. The inhibitory effects of this chemical on contractile responses induced by AII persists after the strips are washed. [1] It suppresses the increase in plasma glucose level in the oral glucose tolerance test (OGTT) without significant change in insulin concentration and improved insulin sensitivity. In skeletal muscle, this agent decreases the expression of TNF-α at doses of 0.001%. In adipose tissue, it reduces TNF-α expression but increases the expression of adiponectin, PPARγ, C/EBα, and aP2. [2] In cultured 3T3-L1 preadipocytes, this compound enhances adipogenesis and exertes greater effects than valsartan on expression of genes encoding peroxisome proliferator-activated receptor-α (PPARα), PPARδ, leptin, adipsin, and adiponectin. It also potently inhibits vascular cell proliferation in the absence of exogenously supplemented angiotensin II. [3] | ||
| In vivo | In Koletsky rats, Azilsartan treatment lowers blood pressure, basal plasma insulin concentration and the homeostasis model assessment of insulin resistance index, and inhibited over-increase of plasma glucose and insulin concentrations during oral glucose tolerance test. This compound downregulates 11β-hydroxysteroid dehydrogenase type 1 expression. [4] | ||
| Features | A potent, orally active and specific AII receptor antagonist. |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[1] |
|
| Animal Study:[4] |
|
References
|
Customer Product Validation
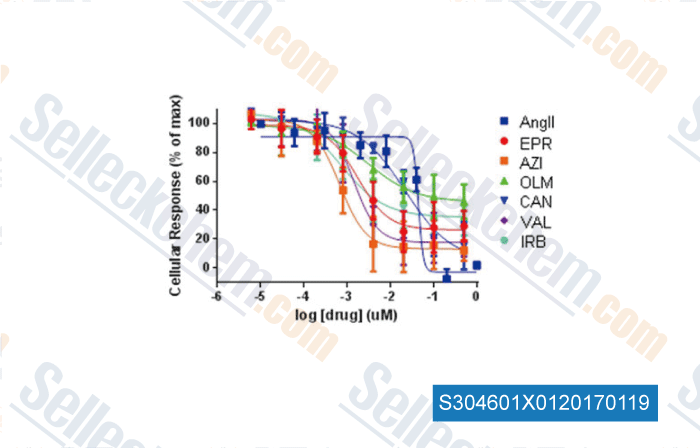
-
Data from [ , , Scientific Reports, 2015, 5:8116. ]
Selleck's Azilsartan Has Been Cited by 4 Publications
| Propafenone facilitates mitochondrial-associated ferroptosis and synergizes with immunotherapy in melanoma [ J Immunother Cancer, 2024, 12(11)e009805] | PubMed: 39581704 |
| Azilsartan Suppresses Osteoclastogenesis and Ameliorates Ovariectomy-Induced Osteoporosis by Inhibiting Reactive Oxygen Species Production and Activating Nrf2 Signaling [ Front Pharmacol, 2021, 12:774709] | PubMed: 34899338 |
| Degradation of SARS-CoV-2 receptor ACE2 by tobacco carcinogen-induced Skp2 in lung epithelial cells [ bioRxiv, 2020, 10.1101/2020.10.13.337774] | PubMed: None |
| Suppression of adrenal barrestin3-dependent aldosteroneproduction by ARBs: head-to-head comparison [Dabul S, et al. Sci Rep, 2015, 5:8116] | PubMed: 25631300 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
