|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C26H33N5O3 |
|||
| Molecular Weight | 463.57 | CAS No. | 1035270-39-3 | |
| Solubility (25°C)* | In vitro | DMSO | 93 mg/mL (200.61 mM) | |
| Ethanol | 40 mg/mL (86.28 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Fexagratinib (AZD4547,ABSK 091) is a novel selective FGFR inhibitor targeting FGFR1/2/3 with IC50 of 0.2 nM/2.5 nM/1.8 nM in cell-free assays, weaker activity against FGFR4, VEGFR2(KDR), and little activity observed against IGFR, CDK2, and p38. Phase 2/3. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||
| In vitro | Compared to FGFR1-3, AZD4547 displays weaker activity against FGFR4 with IC50 of 165 nM. AZD4547 only inhibits recombinant VEGFR2 (KDR) kinase activity with IC50 of 24 nM, in the in vitro selectivity test against a diverse panel of representative human kinases. AZD4547 at 0.1 μM exhibits no activity against a range of recombinant kinases including ALK, CHK1, EGFR, MAPK1, MEK1, p70S6K, PDGFR, PKB, Src, Tie2, and PI3-kinase. Consistently, the potent selectivity of AZD4547 for FGFR1-3 over FGFR4, IGFR, and KDR is also observed in cellular phosphorylation assays. AZD4547 has potent in vitro antiproliferative activity only against tumor cell lines expressing deregulated FGFRs such as KG1a, Sum52-PE, and KMS11 with IC50 of 18-281 nM, and is inactive against MCF7 as well as more than 100 additional tumor cell lines. AZD4547 treatment potently inhibits FGFR and MAPK phosphorylation in human tumor cell lines in a dose-dependent manner. AZD4547 also potently inhibits the phosphorylation of FRS2 and PLCγ, downstream markers of FGFR signaling. Notably, AZD4547 affects the AKT phosphorylation in the breast cell lines, MCF7 and Sum52-PE but not in KG1a and KMS11 lines. AZD4547 treatment significantly induces apoptosis in Sum52-PE and KMS11 cells, dramatically increases G1 arrest but not apoptosis in KG1a cells, and has no effect on cell cycle distribution or apoptosis in MCF7 cells. [1] |
||||||||
| In vivo | Oral administration of AZD4547 at 3 mg/kg twice daily in mice bearing KMS11 tumors results in significant tumor growth inhibition of 53% when compared with vehicle-treated controls, and AZD4547 at 12.5 mg/kg once daily or 6.25 mg/kg twice daily leads to complete tumor stasis, which is associated with dose proportional pharmacodynamic modulation of phospho-FGFR3 and reduced KMS11 tumor cell proliferation. Moreover, oral administration of AZD4547 at 12.5 mg/kg once daily results in 65% tumor growth inhibition in the FGFR1-fusion KG1a xenograft model. At efficacious dose levels, AZD4547 does not exhibit antiangiogenic effects. AZD4547 has no significant effect on blood pressure and therefore lacks in vivo anti-KDR activity. Consistently, dosing of 6.25 mg/kg orally twice daily AZD4547 is inactive in the cediranib-sensitive xenograft models including Calu-6, HCT-15 and LoVo. [1] |
||||||||
| Features | Greater selectivity for FGFR1-3 over FGFR4. AZD4547 is active against the tyrosine kinase activity of both the wild-type and mutant forms of FGFR. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
Customer Product Validation
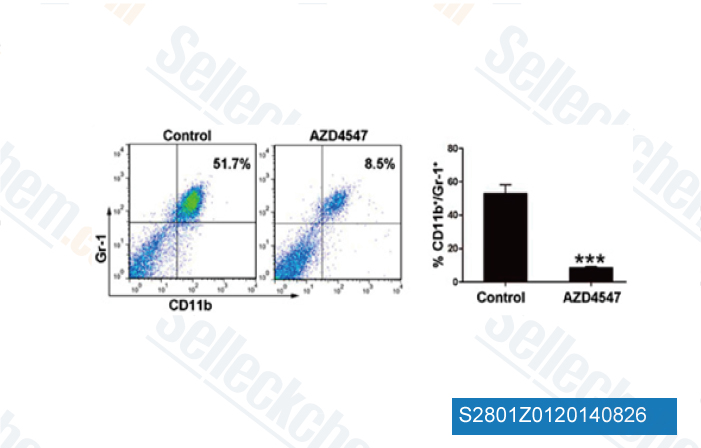
-
Data from [Cell Physiol Biochem, 2014, 33(3), 633-45]
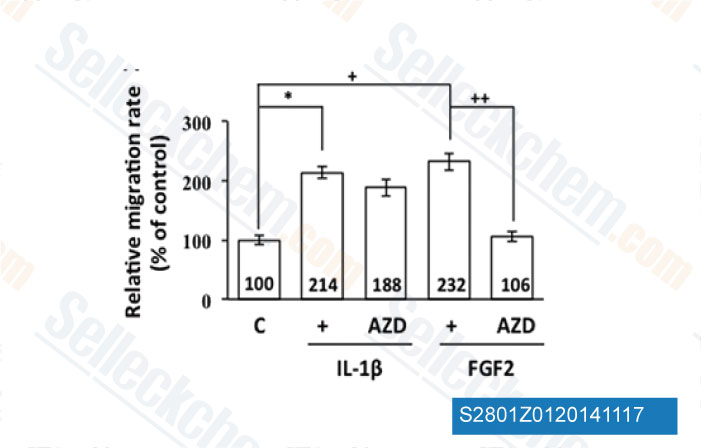
-
Data from [Data independently produced by Mol Cell Biol, 2014, 34(18), 3535-45]
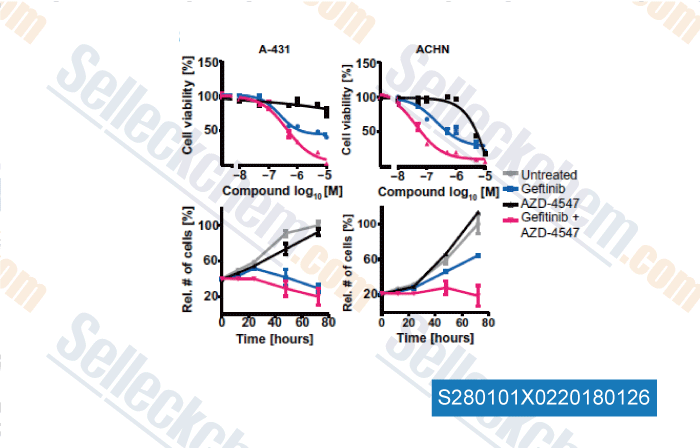
-
, , Science, 2017, doi: 10.1126/science.aan4368
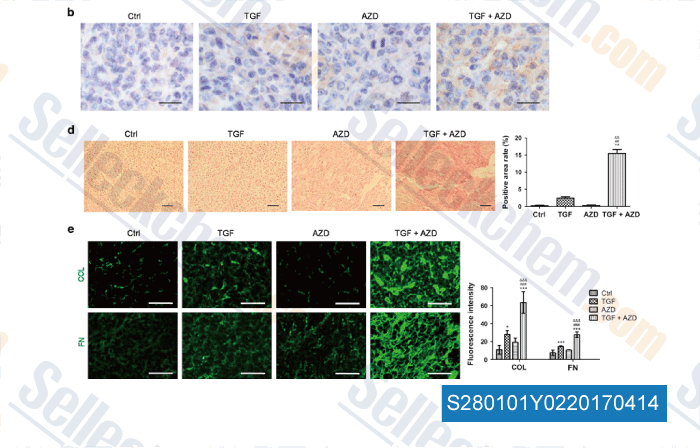
-
, , Oncogene, 2017, 36(27):3831-3841
Selleck's Fexagratinib (AZD4547) has been cited by 149 publications
| Circulating tumor cell plasticity determines breast cancer therapy resistance via neuregulin 1-HER3 signaling [ Nat Cancer, 2025, 6(1):67-85] | PubMed: 39753722 |
| aYAP1-2 contributes to bFGF-induced proliferation In gastric cancer [ Anticancer Drugs, 2025, 36(2):97-103] | PubMed: 39625344 |
| Hyperglycemia activates FGFR1 via TLR4/c-Src pathway to induce inflammatory cardiomyopathy in diabetes [ Acta Pharm Sin B, 2024, 14(4):1693-1710] | PubMed: 38572108 |
| Understanding and Overcoming Resistance to Selective FGFR inhibitors Across FGFR2-Driven Malignancies [ Clin Cancer Res, 2024, 10.1158/1078-0432.CCR-24-1834] | PubMed: 39226398 |
| FKBP10 promotes clear cell renal cell carcinoma progression and regulates sensitivity to the HIF2α blockade by facilitating LDHA phosphorylation [ Cell Death Dis, 2024, 15(1):64] | PubMed: 38233415 |
| FGF receptors mediate cellular senescence in the cystic fibrosis airway epithelium [ JCI Insight, 2024, 9(15)e174888] | PubMed: 38916962 |
| FGF receptor kinase inhibitors exhibit broad antiviral activity by targeting Src family kinases [ Cell Mol Life Sci, 2024, 81(1):471] | PubMed: 39621133 |
| Paradoxical cancer cell proliferation after FGFR inhibition through decreased p21 signaling in FGFR1-amplified breast cancer cells [ Breast Cancer Res, 2024, 26(1):54] | PubMed: 38553760 |
| Anti-inflammatory and remyelinating effects of fexagratinib in experimental multiple sclerosis [ Br J Pharmacol, 2024, 10.1111/bph.17341] | PubMed: 39367768 |
| Kinase inhibitor pulldown assay (KiP) for clinical proteomics [ Clin Proteomics, 2024, 21(1):3] | PubMed: 38225548 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
