|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C25H29I2NO3.HCl |
|||
| Molecular Weight | 681.77 | CAS No. | 19774-82-4 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (146.67 mM) | |
| Ethanol | 20 mg/mL (29.33 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Amiodarone HCl is a sodium/potassium-ATPase inhibitor and an autophagy activator, used to treat various types of cardiac dysrhythmias. | |
|---|---|---|
| Targets |
|
|
| In vitro | Amiodarone possesses an inhibitory effect on the fast sodium channel as well as on the slow calcium channel. Amiodarone also has non-competitive antisympathetic effects, and modulates thyroid function and phospholipid metabolism. Amiodarone penetrates deeply into the lipid matrix of the membrane, and is released from cardiac tissues very slowly when washed out. Amiodarone (44–88 μM) depresses Vmax of guinea pig papillary muscle without affecting the resting membrane potential, and that this Vmax inhibition is enhanced in a frequency- or use-dependent manner like Class I antiarrhythmic drugs. Amiodarone (50–88 μM) is also found to suppress the depolarization-induced spontaneous action potentials (abnormal automaticity) in ventricular muscles and in Purkinje fibers. [1] |
|
| In vivo | Amiodarone (1.25–25 mg/kg) results in a decrease in sinus rate, a prolongation of effective and functional refractory periods of the atrioventricular node, and a frequency-dependent conduction delay in the atrioventricular node and in the ventricle of anesthetized dogs. Amiodarone (50 mg/kg/day, i.p. for 3–4 weeks) results in significant decreases in the current density of iK and ito in ventricular cells without affecting iCa and iK1 densities in rabbit. Amiodarone (AM) inhibits intracellular conversion from thyroxine (T4) to triiodothyronine (T3) via 5′-deiodination (5′DI) without affecting intracellular conversion from T4 to reverse T3 (rT3). [1] |
Protocol (from reference)
Customer Product Validation
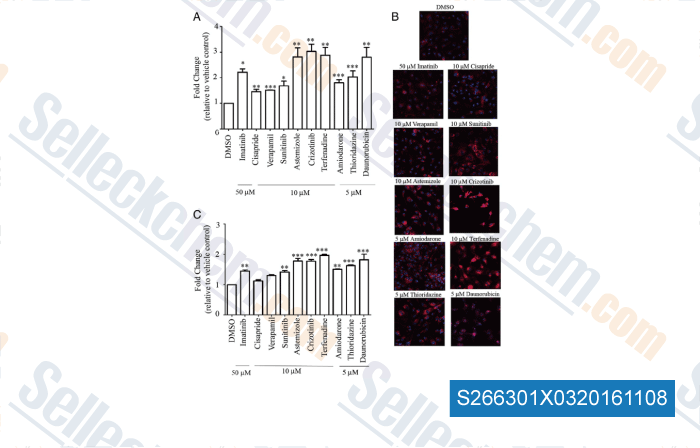
-
Data from [Data independently produced by , , Toxicol Appl Pharmacol, 2015, 285(1):51-60.]
Selleck's Amiodarone HCl has been cited by 13 publications
| Propafenone facilitates mitochondrial-associated ferroptosis and synergizes with immunotherapy in melanoma [ J Immunother Cancer, 2024, 12(11)e009805] | PubMed: 39581704 |
| Quantitative chemometric phenotyping of three-dimensional liver organoids by Raman spectral imaging [ Cell Rep Methods, 2023, 3(4):100440] | PubMed: 37159662 |
| Quantitative chemometric phenotyping of three-dimensional liver organoids by Raman spectral imaging [ Cell Rep Methods, 2023, 3(4):100440] | PubMed: 37159662 |
| Quantitative chemometric phenotyping of three-dimensional liver organoids by Raman spectral imaging [ Cell Reports Methods, 2023, 10.1016/j.crmeth.2023.100440] | PubMed: None |
| Dextran sodium sulfate potentiates NLRP3 inflammasome activation by modulating the KCa3.1 potassium channel in a mouse model of colitis [ Cell Mol Immunol, 2022, 19(8):925-943] | PubMed: 35799057 |
| Moderate heart rate reduction promotes cardiac regeneration through stimulation of the metabolic pattern switch [ Cell Rep, 2022, 38(10):110468] | PubMed: 35263588 |
| KCNQ1-deficient and KCNQ1-mutant human embryonic stem cell-derived cardiomyocytes for modeling QT prolongation [ Stem Cell Res Ther, 2022, 13(1):287] | PubMed: 35765105 |
| Eryptosis and Malaria: New Experimental Guidelines and Re-Evaluation of the Antimalarial Potential of Eryptosis Inducers [ Front Cell Infect Microbiol, 2021, 11:630812] | PubMed: 33777843 |
| Strain-Dependent Variability of Early Discovery Small Molecule Pharmacokinetics in Mice: Does Strain Matter? [ Drug Metab Dispos, 2020, 48(8):613-621] | PubMed: 32474442 |
| Quantifying Drug Combination Synergy along Potency and Efficacy Axes. [ Cell Syst, 2019, 8(2):97-108] | PubMed: 30797775 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
