|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C16H24N2O4 |
|||
| Molecular Weight | 308.37 | CAS No. | 58970-76-6 | |
| Solubility (25°C)* | In vitro | DMSO | 5 mg/mL (16.21 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Bestatin (Ubenimex) is a potent aminopeptidase-B and leukotriene (LT) A4 hydrolase inhibitor, used in the treatment of acute myelocytic leukemia. | |
|---|---|---|
| Targets |
|
|
| In vitro | Bestatin inhibits proliferation of all the human leukemic cell lines except KG1. Bestatin induces DNA fragmentation quantitatively and DNA ladder and enhances caspase-3 activity in U937 cells. Bestatin dose-dependently induces DNA fragmentation in human leukemic cell lines. [1] Bestatin dose-dependently inhibits the invasion of SN12M cells into reconstituted basement membrane (Matrigel). Bestatin inhibits the degradation of type IV collagen by tumor cells, but not by tumor-conditioned medium (TCM), in a concentration-dependent manner. Bestatin inhibits hydrolysing activities towards substrates of aminopeptidases in SN12M cells. [2] Bestatin inhibits the tube-like formation of human umbilical vein endothelial cells (HUVECs) in vitro. [3] Bestatin exerts a direct stimulating effect on lymphocytes (and monocytes) via its fixation on cell surface leucine-aminopeptidase, and an indirect effect on monocytes (and lymphocytes) via aminopeptidase B inhibition of tuftsin catabolism. [4] |
|
| In vivo | Bestatin significantly inhibits the melanoma cell-induced angiogenesis in a mouse dorsal air sac assay. Bestatin reduces the number of vessels oriented towards the established primary tumor mass on the dorsal side of mice implantated of B16-BL6 melanoma cells. [3] Bestatin statistically significantly inhibits leukotriene B4 biosynthesis in the esophageal tissues of EGDA rats and reduces the incidence of EAC in the EGDA rats from 57.7% (15 of 26 rats) to 26.1% (6 of 23 rats). [5] |
Protocol (from reference)
References
Customer Product Validation
-
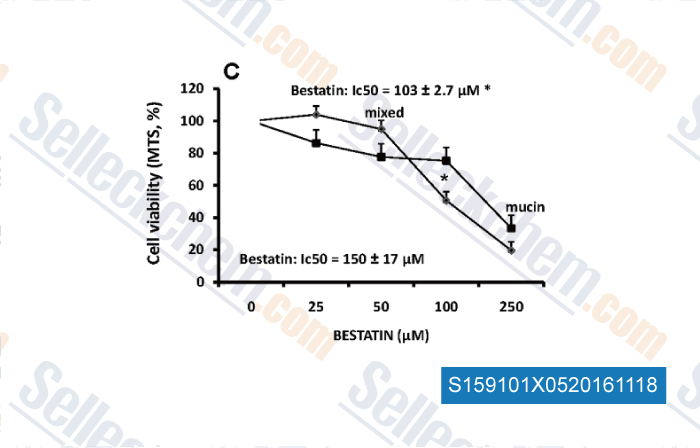
Data from [Data independently produced by , , PLoS One, 2015, 10(11):e0142124.]
Selleck's Bestatin (Ubenimex) has been cited by 9 publications
| Targeting the GPI transamidase subunit GPAA1 abrogates the CD24 immune checkpoint in ovarian cancer [ Cell Rep, 2024, 43(4):114041] | PubMed: 38573857 |
| Novel paired CD13-negative (MT-50.1) and CD13-positive (MT-50.4) HTLV-1-infected T-cell lines with differential regulatory T cell-like activity [ Sci Rep, 2024, 14(1):12549] | PubMed: 38822041 |
| Protocol for monitoring phagocytosis of cancer cells by TAM-like macrophages using imaging cytometry [ STAR Protoc, 2024, 5(4):103320] | PubMed: 39298319 |
| Cholesterol-induced leucine aminopeptidase 3 (LAP3) upregulation inhibits cell autophagy in pathogenesis of NAFLD [ Aging (Albany NY), 2022, 14(7):3259-3275] | PubMed: 35404840 |
| Sarsasapogenin ameliorates diabetes-associated memory impairment and neuroinflammation through down-regulation of PAR-1 receptor [ Phytother Res, 2021, 35(6):3167-3180] | PubMed: 33885189 |
| CD13 promotes hepatocellular carcinogenesis and sorafenib resistance by activating HDAC5-LSD1-NF-κB oncogenic signaling [ Clin Transl Med, 2020, 10(8):e233] | PubMed: 33377659 |
| Sarsasapogenin alleviates diabetic nephropathy through suppression of chronic inflammation by down-regulating PAR-1: In vivo and in vitro study [ Phytomedicine, 2020, 78:153314] | PubMed: 32882582 |
| Thrombin/PAR-1 activation induces endothelial damages via NLRP1 inflammasome in gestational diabetes [ Biochem Pharmacol, 2020, 175:113849] | PubMed: 32059841 |
| Sensitivity of Human Intrahepatic Cholangiocarcinoma Subtypes to Chemotherapeutics and Molecular Targeted Agents: A Study on Primary Cell Cultures [Fraveto A, et al. PLoS One, 2015, 10(11):e0142124] | PubMed: 26571380 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
