|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C29H39N5O8 |
||||||
| Molecular Weight | 585.65 | CAS No. | 220620-09-7 | ||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (170.75 mM) | ||||
| Water | 100 mg/mL (170.75 mM) | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Tigecycline is bacteriostatic and is a protein synthesis inhibitor by binding to the 30S ribosomal subunit of bacteria and thereby blocking entry of Aminoacyl-tRNA into the A site of the ribosome during prokaryotic translation. Tigecycline induces autophagy by downregulating the PI3K-AKT-mTOR pathway. |
|---|---|
| In vitro | Tigecycline evades the Tet(A-E) efflux pumps, which account for most acquired resistance to tetracycline and minocycline in Enterobacteriaceae and Acinetobacter spp. Tigecycline binds to bacterial ribosomes that have been modified by the Tet(M) protein, a mechanism that compromises all available tetracyclines, and which is frequent in Gram-positive cocci and Neisseria spp. Tigecycline remains vulnerable to the chromosomally-encoded multidrug efflux pumps of Proteeae and Pseudomonas aeruginosa, and to Tet(X), a tetracycline-degrading mono-oxygenase found, albeit rarely, in Bacteroides spp. Tigecycline MICs for enterococci, staphylococci, and streptococci are mostly 0.06–0.25 mg/L, again with little or no skew to the distribution. Tigecycline is prone to oxidation, and MIC values, particularly for the most susceptible isolates, may be raised if the drug is added to broth that has become oxygenated during storage, or if drug-containing media are stored before inoculation. [1] Tigecycline is a poor substrate for tetracycline-specific efflux pumps, and it still attaches to ribosomes that have been modified by the Tet(M) protein. Tigecycline has demonstrated activity against a wide variety of gram-positive and gram-negative pathogens, including multidrug-resistant strains. Tigecycline is active against many gram-positive and -negative organisms, including methicillin-resistantStaphylococcus aureus, vancomycin-intermediate and -resistant enterococci, and extended-spectrum β-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Tigecycline exhibits antibacterial activity against a wide spectrum of aerobic and anaerobic bacteria. [2] Tigecycline is a broad-spectrum, protein-inhibiting, antibacterial agent possessing activity against strains resistant to other chemotherapeutic agents. Tigecycline demonstrates in vitro activities against the GISA and the methicillin-resistant and methicillin-susceptible staphylococcal strains tested (MICs at which 90% of isolates tested are inhibited [MIC90s], 0.5 to 1 μg/ml). Tigecycline has MIC90s of 0.25 μg/ml for all of the S. pneumoniae strains and demonstrates similar activities against all of the S. pneumoniae strains tested. [3] |
| In vivo | Tigecycline is bactericidal against methicillin-susceptible S. aureus (MSSA) in the rabbit osteomyelitis model and exhibits good, but not excellent, activity in Legionellapneumophila pneumonia in guinea pigs[1]. Tigecycline at 50 mg/kg twice daily was not toxic to mice. Tigecycline is effective in inhibiting NSCLC growth in vivo through decreasing proliferation and increasing apoptosis of tumor cells[4]. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
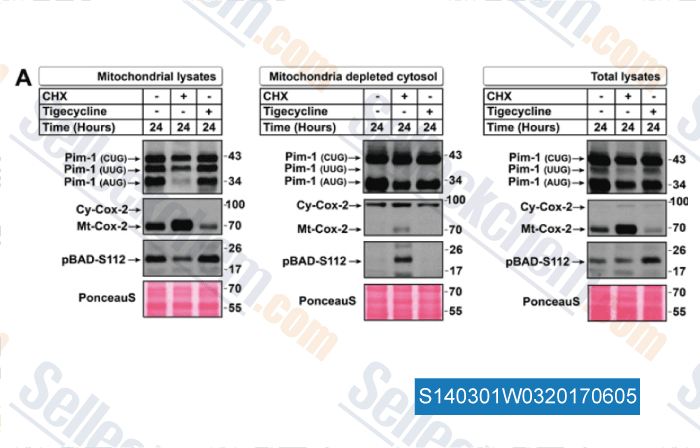
-
Data from [Data independently produced by , , Biochem J, 2016, 473(1):99-107]
Selleck's Tigecycline has been cited by 16 publications
| Flash optimization of drug combinations for Acinetobacter baumannii with IDentif.AI-AMR [ NPJ Antimicrob Resist, 2025, 3(1):12] | PubMed: 39984645 |
| Tigecycline Opposes Bortezomib Effect on Myeloma Cells Decreasing Mitochondrial Reactive Oxygen Species Production [ Int J Mol Sci, 2024, 25(9)4887] | PubMed: 38732105 |
| Tigecycline Opposes Bortezomib Effect on Myeloma Cells Decreasing Mitochondrial Reactive Oxygen Species Production [ Int J Mol Sci, 2024, 25(9)4887] | PubMed: 38732105 |
| Meropenem-ANT3310, a unique β-lactam-β-lactamase inhibitor combination with expanded antibacterial spectrum against Gram-negative pathogens including carbapenem-resistant Acinetobacter baumannii [ Antimicrob Agents Chemother, 2024, 68(3):e0112023] | PubMed: 38289044 |
| Sustained activation of EGFR-ERK1/2 signaling limits the response to tigecycline-induced mitochondrial respiratory deficiency in liver cancer [ EBioMedicine, 2022, 87:104397] | PubMed: 36502574 |
| Activity of Drug Combinations against Mycobacterium abscessus Grown in Aerobic and Hypoxic Conditions [ Microorganisms, 2022, 10(7)1421] | PubMed: 35889140 |
| The long non-coding RNA CDK6-AS1 overexpression impacts on acute myeloid leukemia differentiation and mitochondrial dynamics [ iScience, 2021, 24(11):103350] | PubMed: 34816103 |
| Targeting Mitochondrial Metabolism in Clear Cell Carcinoma of the Ovaries [ Int J Mol Sci, 2021, 22(9)4750] | PubMed: 33947138 |
| Targeting Mitochondria in Melanoma [ Biomolecules, 2020, 10(10)E1395] | PubMed: 33007949 |
| Sirt1 gene confers Adriamycin resistance in DLBCL via activating the PCG-1α mitochondrial metabolic pathway [ Aging (Albany NY), 2020, 12(12):11364-11385] | PubMed: 32570218 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
