|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C13H10N2O4 |
|||
| Molecular Weight | 258.23 | CAS No. | 50-35-1 | |
| Solubility (25°C)* | In vitro | DMSO | 52 mg/mL (201.37 mM) | |
| Ethanol | 2 mg/mL (7.74 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Thalidomide was introduced as a sedative drug, immunomodulatory agent and also is investigated for treating symptoms of many cancers. Thalidomide inhibits cereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Thalidomide must be metabolized by the liver to form an epoxide that may be the active teratogenic metabolite. [1] Thalidomide selectively inhibits the production of human monocyte tumor necrosis factor alpha (TNF-alpha) when human monocytes are triggered with lipopolysaccharide and other agonists in culture. [2] Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. [3] Thalidomide acts directly, by inducing apoptosis or G1 growth arrest, in MM cell lines and in patient MM cells that are resistant to melphalan, doxorubicin, and dexamethasone (Dex). Thalidomide enhances the anti-MM activity of Dex and, conversely, are inhibited by interleukin 6. [4] Thalidomide is a potent costimulator of primary human T cells in vitro, synergizing with stimulation via the T cell receptor complex to increase interleukin 2-mediated T cell proliferation and interferon gamma production. Thalidomide also increases the primary CD8+ cytotoxic T cell response induced by allogeneic dendritic cells in the absence of CD4+ T cells. [5] |
||
| In vivo | Thalidomide (200 mg/kg) results in an inhibition of the area of vascularized cornea of rabbits that ranged from 30% to 51% in three experiments with a median inhibition of 36%. [1] |
Protocol (from reference)
References
Customer Product Validation
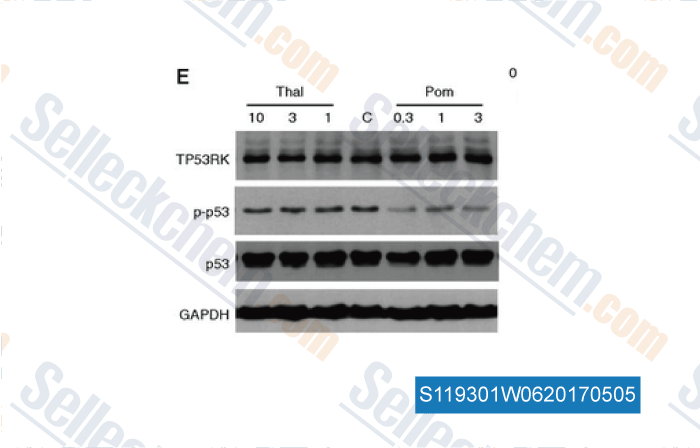
-
Data from [Data independently produced by , , Blood, 2017, 129(10):1308-1319]
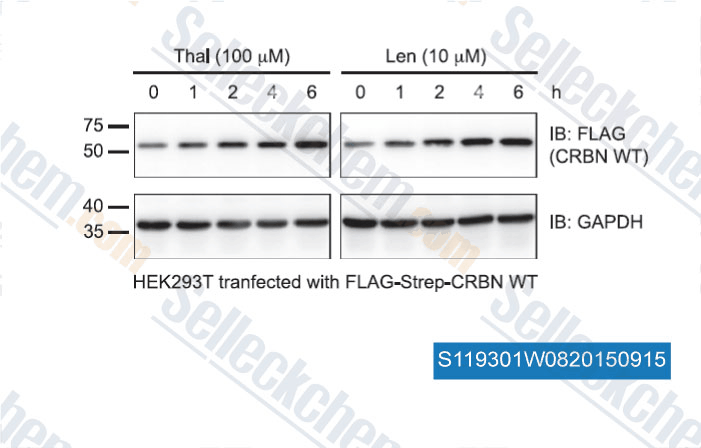
-
Data from [Data independently produced by , , FASEB Journal, 2015, fj.15-274050]
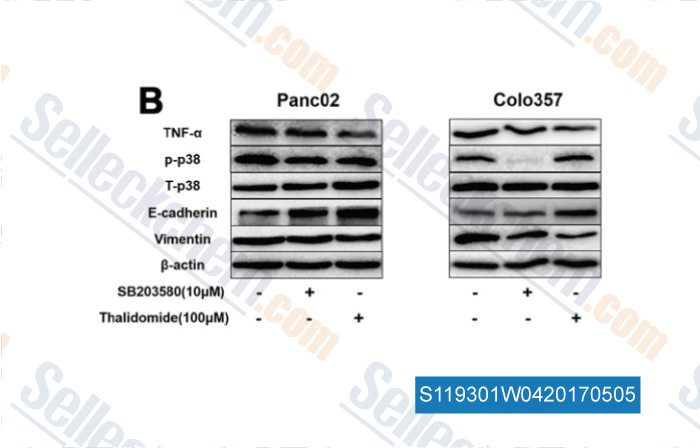
-
Data from [Data independently produced by , , Oncotarget, 2016, 7(25):38539-38550]
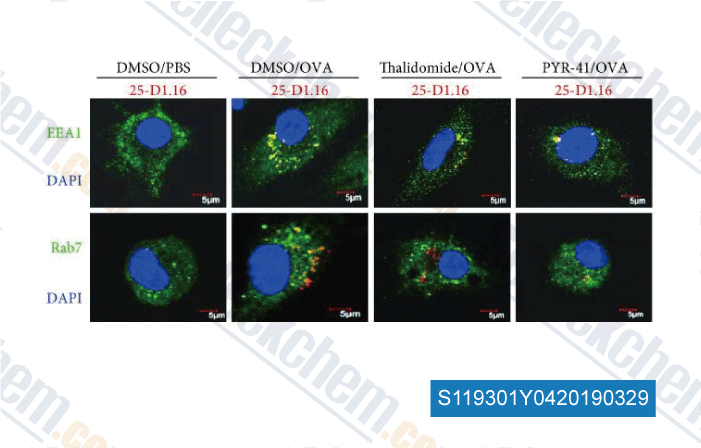
-
Data from [Data independently produced by , , J Immunol Res, 2018, 2018:5070573]
Selleck's Thalidomide has been cited by 38 publications
| Enhancing T cell cytotoxicity in multiple myeloma with bispecific αPD-L1 × αCD3 T cell engager-armed T cells and low-dose bortezomib therapy [ Biomed Pharmacother, 2025, 184:117878] | PubMed: 39891948 |
| Stepwise phosphorylation and SUMOylation of PIDD1 drive PIDDosome assembly in response to DNA repair failure [ Nat Commun, 2024, 15(1):9195] | PubMed: 39448602 |
| Development of an orally bioavailable CDK12/13 degrader and induction of synthetic lethality with AKT pathway inhibition [ Cell Rep Med, 2024, 5(10):101752] | PubMed: 39353441 |
| A small-molecule degrader selectively inhibits the growth of ALK-rearranged lung cancer with ceritinib resistance [ iScience, 2024, 27(2):109015.] | PubMed: 38327793 |
| p300/CBP degradation is required to disable the active AR enhanceosome in prostate cancer [ bioRxiv, 2024, 2024.03.29.587346] | PubMed: 38586029 |
| Protocol to generate cardiac pericytes from human induced pluripotent stem cells [ STAR Protoc, 2023, 4(2):102256] | PubMed: 37119139 |
| Lenalidomide bypasses CD28 co-stimulation to reinstate PD-1 immunotherapy by activating Notch signaling [ Cell Chem Biol, 2022, S2451-9456(22)00204-5] | PubMed: 35732177 |
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
| Establishment and characterization of NCC-UPS4-C1: a novel cell line of undifferentiated pleomorphic sarcoma from a patient with Li-Fraumeni syndrome [ Hum Cell, 2022, 10.1007/s13577-022-00671-y] | PubMed: 35118583 |
| Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00639-4] | PubMed: 34731453 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
