|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C19H30N5O10P.C4H4O4 |
||||||||||
| Molecular Weight | 635.51 | CAS No. | 202138-50-9 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (157.35 mM) | ||||||||
| Ethanol | 4 mg/mL (6.29 mM) | ||||||||||
| Water | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Tenofovir Disoproxil Fumarate belongs to a class of antiretroviral drugs, it inhibits the activity of HIV reverse transcriptase by competing with the natural substrate deoxyadenosine 5’-triphosphate and, after incorporation into DNA, by DNA chain termination. | |
|---|---|---|
| Targets |
|
|
| In vitro | Tenofovir is eliminated from systemic circulation renally through a combination of glomerular filtration and active tubular secretion. Tenofovir is not a substrate for human organic cation transporter type 1 (hOCT1) or hOCT2. Tenofovir accumulates to fivefold lower levels in MRP4-overexpressing cells, and its accumulation could be increased by an MRP inhibitor. [1] Tenofovir produces no significant changes in mitochondrial DNA (mtDNA) levels in human hepatoblastoma (HepG2) cells, skeletal muscle cells (SkMCs), or renal proximal tubule epithelial cells. Tenofovir elevates lactate production by less than 20% in HepG2 cells or SkMCs. [2] Tenofovir is efficiently phosphorylated to tenofovir diphosphate (TFV-DP) in both HepG2 cells and primary human hepatocytes. Tenofovir has a 50% effective concentration of 1.1 mM against HBV in cell-based assays, and potency is improved > 50-fold by the addition of bis-isoproxil progroups. Tenofovir has previously demonstrated full activity against lamivudine-resistant HBV in vitro and clinically. [3] Tenofovir inhibits the proliferation of liver-derived HepG2 cells and normal skeletal muscle cells with CC(50) values of 398 μM and 870 μM, respectively. Tenofovir shows substantially weaker effects on proliferation and viability of renal proximal tubule epithelial cells than cidofovir, a related nucleotide analog with the potential to induce renal tubular dysfunction. [4] |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
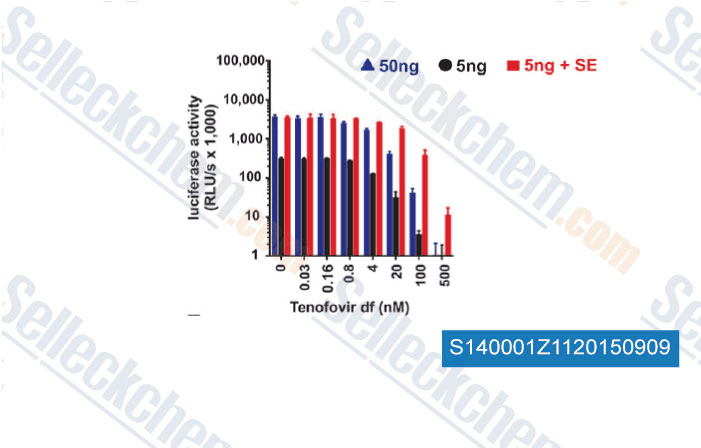
-
Data from [Data independently produced by , , Sci Transl Med, 2014, 6(262): 262ra157 ]
Selleck's Tenofovir Disoproxil Fumarate has been cited by 29 publications
| IKAROS expression drives the aberrant metabolic phenotype of macrophages in chronic HIV infection [ Clin Immunol, 2024, 260:109915] | PubMed: 38286172 |
| Preclinical and clinical antiviral characterization of AB-836, a potent capsid assembly modulator against hepatitis B virus [ Antiviral Res, 2024, 231:106010] | PubMed: 39326502 |
| Broad synergistic antiviral efficacy between a novel elite controller-derived dipeptide and antiretrovirals against drug-resistant HIV-1 [ Front Cell Infect Microbiol, 2024, 14:1334126] | PubMed: 38915925 |
| Low-dose treatment with Epirubicin, a novel histone deacetylase 1 inhibitor, exerts anti-leukemic effects by inducing ferroptosis [ Eur J Pharmacol, 2024, 985:177058] | PubMed: 39413949 |
| Preclinical Antiviral and Safety Profiling of the HBV RNA Destabilizer AB-161 [ Viruses, 2024, 16(3)323] | PubMed: 38543689 |
| Acute antagonism in three-drug combinations for vaginal HIV prevention in humanized mice [ Sci Rep, 2023, 13(1):4594] | PubMed: 36944714 |
| Establishment and characterization of a new cell culture system for hepatitis B virus replication and infection [ Virol Sin, 2022, S1995-820X(22)00081-5] | PubMed: 35568375 |
| Reduction of CD8 T cell functionality but not inhibitory capacity by integrase inhibitors [ J Virol, 2022, JVI0173021] | PubMed: 35019724 |
| Female Genital Fibroblasts Diminish the In Vitro Efficacy of PrEP against HIV [ Viruses, 2022, 14(8)1723] | PubMed: 36016345 |
| Impaired differentiation of small airway basal stem/progenitor cells in people living with HIV [ Sci Rep, 2022, 12(1):2966] | PubMed: 35194053 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
