|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C19H18ClN3O5S |
||||||
| Molecular Weight | 435.88 | CAS No. | 366789-02-8 | ||||
| Solubility (25°C)* | In vitro | DMSO | 87 mg/mL (199.59 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Rivaroxaban is a direct inhibitor of Factor Xa with Ki and IC50 of 0.4 nM and 0.7 nM in cell-free assays, respectively. It is selective for human factor Xa, for which it has >10 000-fold greater selectivity than for other biologically relevant serine proteases (IC50 >20 μM). | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Rivaroxaban is an oral, direct inhibitor of Factor Xa (FXa), being developed for the prevention and treatment of arterial and venous thrombosis with a Ki of 0.4 nM. Rivaroxaban also inhibits prothrombinase activity with IC50 of 2.1 nM. Rivaroxaban also shows a similar affinity to purified human and rabbit FXa (IC50 0.7 nM and 0.8 nM, respectively), but a lesser potency against purified rat FXa (IC50 3.4 nM). Endogenous human and rabbit FXa in plasma is inhibited to a similar extent by Rivaroxaban (IC50 21 nM and 21 nM, respectively), while 14-fold higher concentrations are required in rat plasma (IC50 290 nM). [1] Rivaroxaban exhibits high permeability and polarized transport across Caco-2 cells as a substrate of the P-gp, but exhibits no inhibitory effect on P-gp-mediated drug transport up to concentrations of 100 μM in vitro. [2] | ||||
| In vivo | Rivaroxaban reduces venous thrombosis in a dose dependent manner (ED50 0.1 mg/kg i.v.) in a rat venous stasis model. Rivaroxaban reduces arterial thrombus formation in an arteriovenous (AV) shunt in rats (ED50 5.0 mg/kg p.o.) and rabbits (ED50 0.6 mg/kg p.o.). [1] Plasma pharmacokinetics of Rivaroxaban are linear across the investigated dose range (1-10 mg/kg in rats, 0.3-3 mg/kg in dogs). Plasma clearance is low: 0.4 L/kg/h in rats and 0.3 L/kg/h in dogs; the volume of distribution (V(ss)) is moderate: 0.3 L/kg in rats, and 0.4 L/kg in dogs. The elimination half-life after oral administration is short in both species (0.9-2.3 hours). [3] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
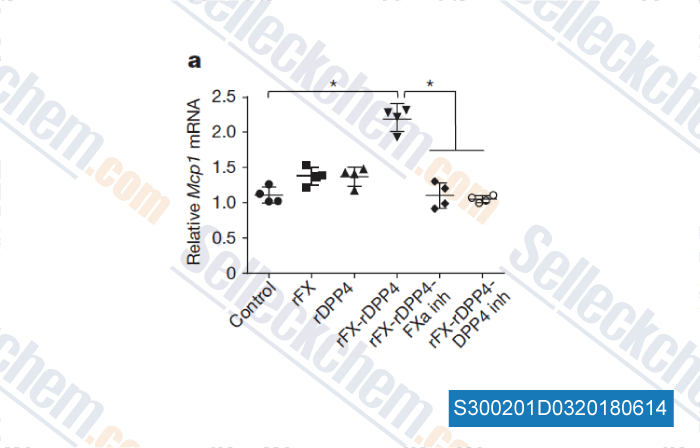
-
Data from [Data independently produced by , , Nature, 2018, 555(7698):673-677]
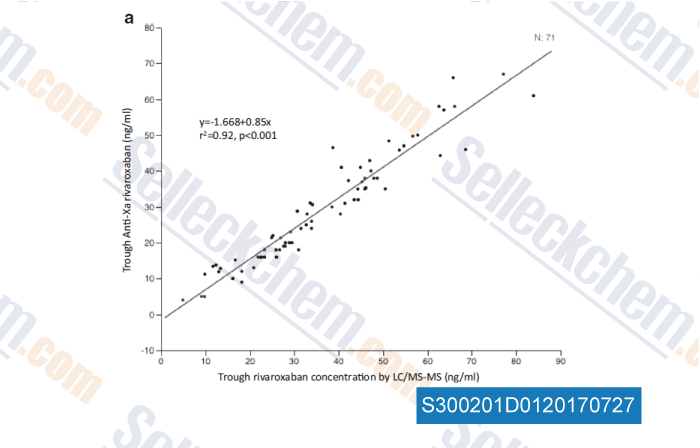
-
Data from [Data independently produced by , , Eur J Clin Pharmacol, 2016, 72(6):671-9 ]
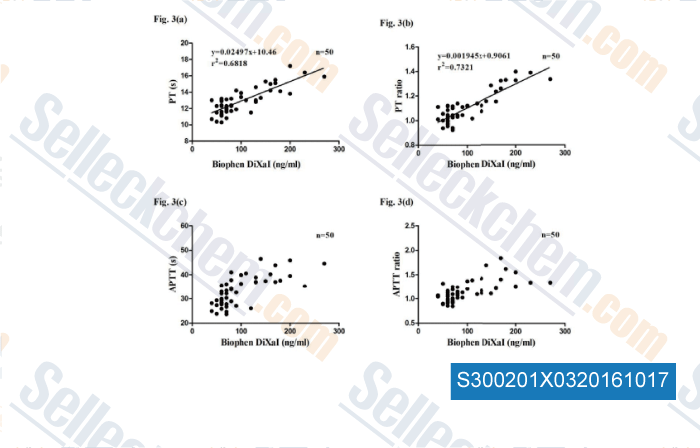
-
Data from [Data independently produced by , , Br J Biomed Sci, 2016, 73(3):134-139.]
Selleck's Rivaroxaban has been cited by 30 publications
| Real-Time Imaging of Platelet-Initiated Plasma Clot Formation and Lysis Unveils Distinct Impacts of Anticoagulants [ Thromb Haemost, 2025, 10.1055/a-2497-4213] | PubMed: 39788528 |
| Coagulation factor X promotes resistance to androgen-deprivation therapy in prostate cancer [ Cancer Cell, 2024, 42(10):1676-1692.e11] | PubMed: 39303726 |
| Engineering and evaluation of FXa bypassing agents that restore hemostasis following Apixaban associated bleeding [ Nat Commun, 2024, 15(1):3912] | PubMed: 38724509 |
| Detection of direct oral anticoagulants with the diluted Russel's viper venom time [ Int J Lab Hematol, 2024, 10.1111/ijlh.14300] | PubMed: 38721750 |
| Rivaroxaban attenuates neutrophil maturation in the bone marrow niche [ Basic Res Cardiol, 2023, 118(1):31] | PubMed: 37580509 |
| OKL-1111, A modified cyclodextrin as a potential universal reversal agent for anticoagulants [ Thromb Res, 2023, 227:17-24] | PubMed: 37207560 |
| pKr-2 induces neurodegeneration via upregulation of microglial TLR4 in the hippocampus of AD brain [ Brain Behav Immun Health, 2023, 28:100593] | PubMed: 36798617 |
| pKr-2 induces neurodegeneration via upregulation of microglial TLR4 in the hippocampus of AD brain [ Brain Behav Immun Health, 2023, 28:100593] | PubMed: 36798617 |
| CM-352 EFFICACY IN A MOUSE MODEL OF ANTICOAGULANT-ASSOCIATED INTRACRANIAL HAEMORRHAGE [ Thromb Haemost, 2022, 10.1055/a-1759-9962] | PubMed: 35114692 |
| Evaluation of Xa inhibitors as potential inhibitors of the SARS-CoV-2 Mpro protease [ PLoS One, 2022, 17(1):e0262482] | PubMed: 35015795 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
