|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C9H13NO2.HCl |
|||
| Molecular Weight | 203.67 | CAS No. | 61-76-7 | |
| Solubility (25°C)* | In vitro | DMSO | 41 mg/mL (201.3 mM) | |
| Water | 41 mg/mL (201.3 mM) | |||
| Ethanol | 41 mg/mL (201.3 mM) | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Phenylephrine HCl is a selective α1-adrenergic receptor agonist, used primarily as a decongestant. | |
|---|---|---|
| Targets |
|
|
| In vitro | Phenylephrine causes a rapid translocation of PKC-epsilon (EC50 = 0.9 mM) but the proportion lost from the soluble fraction is less than with ET-1. [1] Phenylephrine at pCa 7 causes a dose-dependent increase in contractile force of the hyperpermeable cells, which is reversible on addition of phentolamine. [2] Phenylephrine also protects cardiomyocytes against subsequent 24 h treatment with hypoxia and serum deprivation. Phenylephrine prevents the down-regulation of Bcl-2 and Bcl-X mRNA/protein and induces hypertrophic growth. Phenylephrine-mediated protection is abrogated by the phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor wortmannin and is mimicked by the caspase-9 peptidic inhibitor LEHD-fmk. [3] Phenylephrine stimulates phosphoinositide (PI) hydrolysis, cell growth, and expression of several genes [e.g., atrial natriuretic factor (ANF)] often associated with cardiac hypertrophy. [4] Phenylephrine (1 礛) markedly potentiates HGF-induced hepatocyte DNA synthesis and proliferation. [5] Phenylephrine (10 mM) reversibly increases I(Ca,L) (51.3%; n = 40) and shifted peak I(Ca,L) activation voltage by -10 mV. Phenylephrine also increases local, subsarcolemmal SR Ca2+ release via IP3-dependent signaling. Phenylephrin-induced NOi release requires stimulation of both PI-3K/Akt and IP3-dependent Ca2+ signaling. Phenylephrine-induced NOi release is inhibited by each of 1 mM prazocin, 10 mM L-NIO, 10 mM W-7, 10 mM LY294002, 2 mM H-89, 10 mM ryanodine, 5 mM thapsigargin, 2 mM 2-APB or 10 mM xestospongin C. [6] |
Protocol (from reference)
References
Customer Product Validation
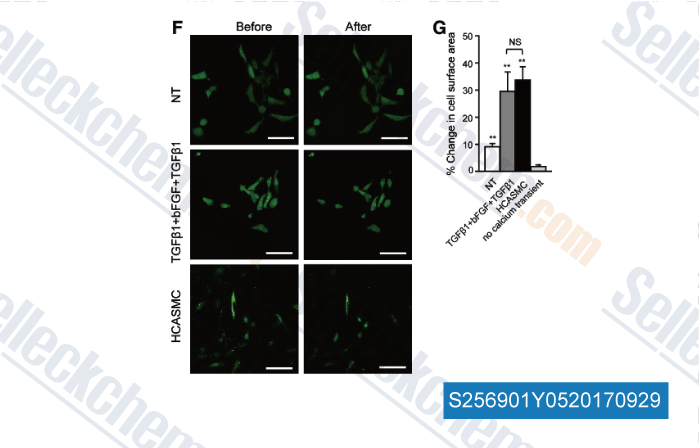
-
Data from [Data independently produced by , , Stem Cells Dev, 2017, 26(7):528-540]
Selleck's Phenylephrine HCl has been cited by 10 publications
| Modulation of Abnormal Vasoconstriction Through 2-Hydroxyisobutyrylation of Tropomyosin 3 Lys141: Targeting Histone Deacetylase 3 as a Key Approach [ J Am Heart Assoc, 2025, 14(1):e037400] | PubMed: 39719422 |
| A modified apical resection model with high accuracy and reproducibility in neonatal mouse and rat hearts [ NPJ Regen Med, 2023, 8(1):9] | PubMed: 36806296 |
| α1-adrenoceptor stimulation ameliorates lipopolysaccharide-induced lung injury by inhibiting alveolar macrophage inflammatory responses through NF-κB and ERK1/2 pathway in ARDS [ Front Immunol, 2022, 13:1090773] | PubMed: 36685596 |
| α1-adrenoceptor stimulation ameliorates lipopolysaccharide-induced lung injury by inhibiting alveolar macrophage inflammatory responses through NF-κB and ERK1/2 pathway in ARDS [ Front Immunol, 2022, 10.3389/fimmu.2022.1090773] | PubMed: 36685596 |
| Rap1GAP Mediates Angiotensin II-Induced Cardiomyocyte Hypertrophy by Inhibiting Autophagy and Increasing Oxidative Stress [ Oxid Med Cell Longev, 2021, 2021:7848027] | PubMed: 33936386 |
| A CRM1 Inhibitor Alleviates Cardiac Hypertrophy and Increases the Nuclear Distribution of NT-PGC-1α in NRVMs [ Front Pharmacol, 2019, 10:465] | PubMed: 31133853 |
| 17β-Estradiol nongenomically induces vascular endothelial H2S release by promoting phosphorylation of cystathionine γ-lyase [ J Biol Chem, 2019, 294(43):15577-15592] | PubMed: 31439665 |
| The immunoproteasome catalytic β5i subunit regulates cardiac hypertrophy by targeting the autophagy protein ATG5 for degradation. [ Sci Adv, 2019, 5(5):eaau0495] | PubMed: 31086810 |
| N‑terminal truncated peroxisome proliferator‑activated receptor‑γ coactivator‑1α alleviates phenylephrine‑induced mitochondrial dysfunction and decreases lipid droplet accumulation in neonatal rat cardiomyocytes. [ Mol Med Rep, 2018, 18(2):2142-2152] | PubMed: 29901150 |
| Efficient Differentiation of TBX18+/WT1+ Epicardial-Like Cells from Human Pluripotent Stem Cells Using Small Molecular Compounds. [Zhao J, et al. Stem Cells Dev, 2017, 26(7):528-540] | PubMed: 27927069 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
