|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C20H24FN5O3 |
|||
| Molecular Weight | 401.43 | CAS No. | 873697-71-3 | |
| Solubility (25°C)* | In vitro | DMSO | 80 mg/mL (199.28 mM) | |
| Ethanol | 6 mg/mL (14.94 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Omecamtiv mecarbil (CK-1827452) is a specific cardiac myosin activator and a clinical drug for left ventricular systolic heart failure. Phase 2. | |
|---|---|---|
| Targets |
|
|
| In vitro | In vitro, Omecamtiv mecarbil selectively activates cardiac myosin by increasing the myosin ATPase rate. [1] In isolated cardiac myocytes, Omecamtiv mecarbil results in increase of myocyte contractility and overcomes of the myosin inhibitor BDM without increasing the calcium transient or inhibiting the PDE pathway. [1] | |
| In vivo | Omecamtiv mecarbil significantly increases fractional shortening starting at 0.4 mM plasma concentrations in SD rats, sham animals and in rats with heart failure. [1] In conscious dogs with myocardial infarction (MI-sHF), Omecamtiv mecarbil leads to a significant increase in wall thickening (25%), stroke volume (44%), cardiac output (22%) and left ventricular (LV) systolic ejection time (26%). In addition, Omecamtiv mecarbil also results in the decreases of some hemodynamic parameters including heart rate, mean left atrial pressure, and LV end-diastolic pressure. In conscious dogs with left ventricular hypertrophy (LVH-sHF), Omecamtiv mecarbil leads to similar and not statistically different effects on hemodynamic parameters. [2] |
Protocol (from reference)
| Animal Study:[1] |
|
|---|
References
|
Customer Product Validation
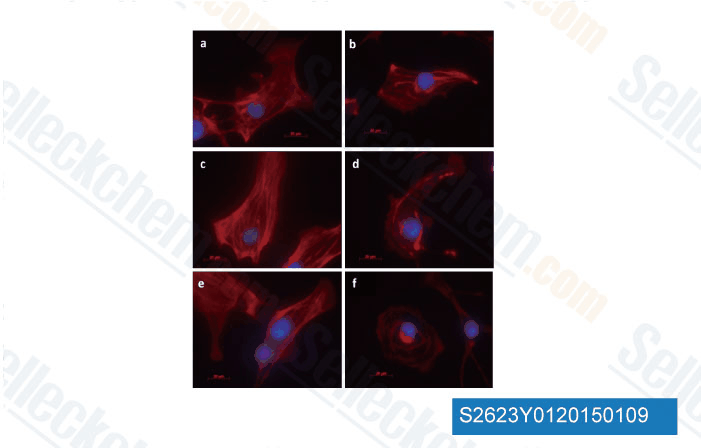
-
Data from [Data independently produced by University of Illinois at Chicago, 2014]
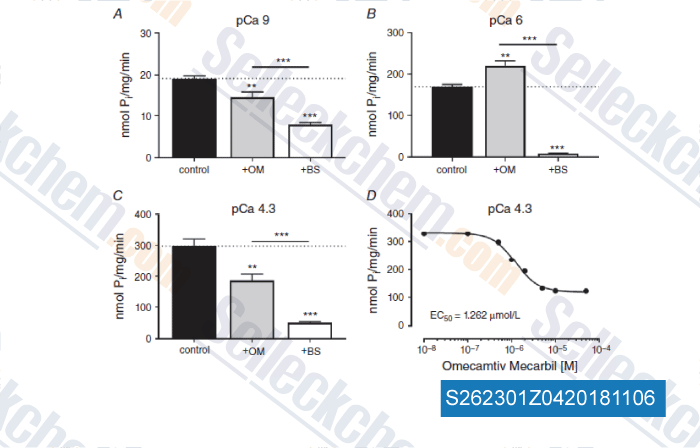
-
Data from [Data independently produced by , , J Physiol, 2018, 596(1):31-46]
Selleck's Omecamtiv mecarbil (CK-1827452) has been cited by 34 publications
| Characterization of NEB pathogenic variants in patients reveals novel nemaline myopathy disease mechanisms and omecamtiv mecarbil force effects [ Acta Neuropathol, 2024, 147(1):72] | PubMed: 38634969 |
| Targeting ErbB and tankyrase1/2 prevent the emergence of drug-tolerant persister cells in ALK-positive lung cancer [ NPJ Precis Oncol, 2024, 8(1):264] | PubMed: 39551860 |
| Deconvolution of polygenic risk score in single cells unravels cellular and molecular heterogeneity of complex human diseases [ bioRxiv, 2024, 2024.05.14.594252] | PubMed: 38798507 |
| Clinically Relevant Models of Cardiac Function using Human Stem Cell-derived Cardiomyocytes [ , 2023, ] | PubMed: none |
| The force of the myosin motor sets cooperativity in thin filament activation of skeletal muscles [ Commun Biol, 2022, 5(1):1266] | PubMed: 36400920 |
| Effects of omecamtiv mecarbil and mavacamten in isolated human atrium [ Naunyn Schmiedebergs Arch Pharmacol, 2022, 10.1007/s00210-022-02333-0] | PubMed: 36399186 |
| Myosin with hypertrophic cardiac mutation R712L has a decreased working stroke which is rescued by omecamtiv mecarbil [ Elife, 2021, 10e63691] | PubMed: 33605878 |
| Mechanistic analysis of actin-binding compounds that affect the kinetics of cardiac myosin-actin interaction [ J Biol Chem, 2021, S0021-9258(21)00245-3] | PubMed: 33639160 |
| Generative adversarial networks for construction of virtual populations of mechanistic models: simulations to study Omecamtiv Mecarbil action [ J Pharmacokinet Pharmacodyn, 2021, 10.1007/s10928-021-09787-4] | PubMed: 34716531 |
| Development and Application of NovelIn VitroAssays to Advance Drug Discovery for Arrhythmogenic Cardiomyopathy and Respiratory Syncytial Virus Infection [ ProQuest , 2021, 28718834] | PubMed: none |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
