|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C6H6N6O2 |
||||||
| Molecular Weight | 194.15 | CAS No. | 85622-93-1 | ||||
| Solubility (25°C)* | In vitro | DMSO | 39 mg/mL (200.87 mM) | ||||
| Water | 5 mg/mL (25.75 mM) | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | TMZ(Temozolomide) is a monofunctional SN-1 alkylating agent that can modify nitrogen atoms in the DNA ring and the extracyclic oxygen group, chemically converted to MTIC and degrades to methyldiazonium cation, which transfers methyl groups to DNA at physiologic pH. A DNA damage inducer in L-1210 and L-1210/BCNU cells. Temozolomide induces apoptosis and exhibits antitumor activity. | |
|---|---|---|
| Targets |
|
|
| In vitro | Methazolastone causes formation of DNA alkali-labile sites which are present in similar amounts and repaired at a similar rate in L-1210 and L-1210/BCNU cell lines. In L-1210 but not in L-1210/BCNU methazolastone induces an arrest of cells in SL-G2-M phases.[1] Methazolastone sensitivity of both chemo-sensitive and resistant cells (D54-R and U87-R) is enhanced significantly under hyperoxia. Both Methazolastone and hyperoxia are associated with increased phosphorylation of ERK p44/42 MAPK (Erk1/2), but to a lesser extent in D54-R cells, suggesting that Erk1/2 activity may be involved in regulation of hyperoxia and Methazolastone-mediated cell death. Hyperoxia enhances Methazolastone toxicity in GBM cells by induction of apoptosis, possibly via MAPK-related pathways. [2] Methazolastone induces in monocytes the DNA damage response pathways ATM-Chk2 and ATR-Chk1 resulting in p53 activation. [3] Chronic Methazolastone exposure results in acquired Methazolastone-resistance and elevates miR-21 expression. [4] Methazolastone treatment triggers endoplasmic reticula (ER) stress with increased expression of GADD153 and GRP78 proteins, and deceases pro-caspase 12 protein. Methazolastone induces autophagy through mitochondrial damage- and ER stress-dependent mechanisms to protect glioma cells. [5] |
|
| In vivo | After a daily i.p. dose of 40 mg/kg for 5 consecutive days (days 1-5 after tumor transplant), methazolastone increases life-span by 86% in L-1210 and 22% in L-1210/BCNU. In L-1210/BCNU no effect is seen after 100 μM or 200 μM treatment; only 400 μM methazolastone produced an accumulation of cells in premitotic phase but much less than in L-1210. In L-1210/BCNU the maximum accumulation of cells in SL-G2-M is, after 48 hours-72 hours, approximately 30% as compared to 23% in untreated cells. Cells accumulates in SL-G2-M occurred too when L- 1210 leukemia-bearing mice are treated i.v. with methazola stone (40 mg/kg). No such effect is seen on L-1210/BCNU cells from mice given the same drug dose. [1] |
|
| Features | Methazolastone is a second-generation alkylating agent. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
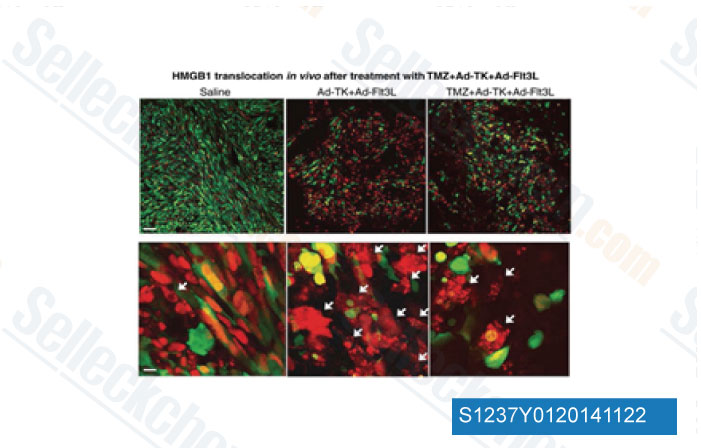
-
Data from [Data independently produced by Clin Cancer Res, 2014, 20(6), 1555-65]
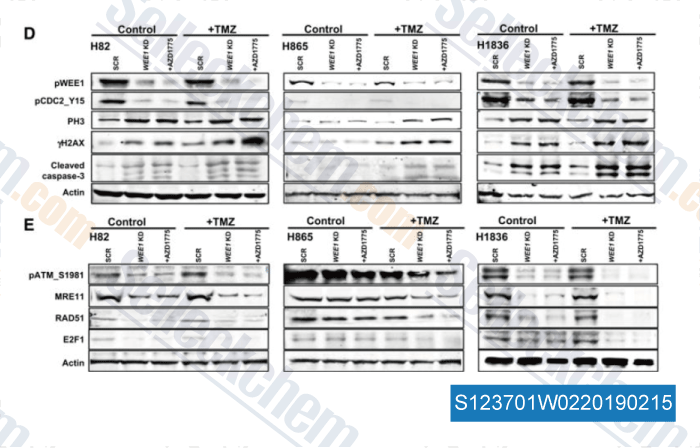
-
Data from [Data independently produced by , , Clin Cancer Res, 2017, 23(20):6239-6253]
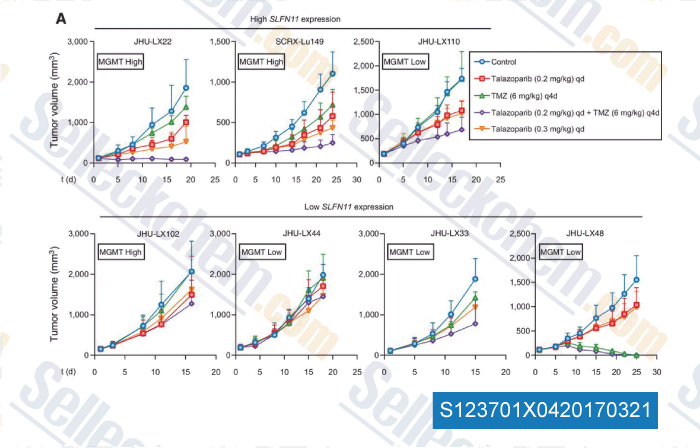
-
Data from [Data independently produced by , , Clin Cancer Res, 2017, 23(2):523-535]
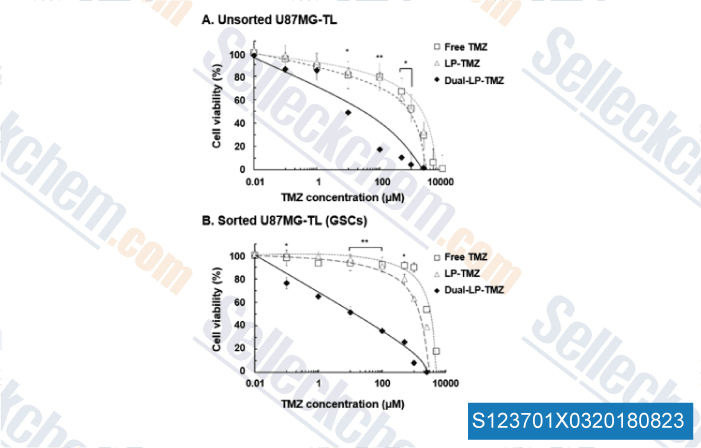
-
Data from [Data independently produced by , , J Control Release, 2018, 269:245-257]
Selleck's TMZ(Temozolomide) has been cited by 241 publications
| Calnexin promotes glioblastoma progression by inducing protective mitophagy through the MEK/ERK/BNIP3 pathway [ Theranostics, 2025, 15(6):2624-2648] | PubMed: 39990231 |
| Overexpression of miR-124 enhances the therapeutic benefit of TMZ treatment in the orthotopic GBM mice model by inhibition of DNA damage repair [ Cell Death Dis, 2025, 16(1):47] | PubMed: 39865088 |
| Transketolase attenuates the chemotherapy sensitivity of glioma cells by modulating R-loop formation [ Cell Rep, 2025, 44(1):115142] | PubMed: 39792560 |
| EZH2 inhibition sensitizes MYC-high medulloblastoma cancers to PARP inhibition by regulating NUPR1-mediated DNA repair [ Oncogene, 2025, 44(6):391-405] | PubMed: 39562655 |
| Imipridones ONC201/ONC206 + RT/TMZ triple (IRT) therapy reduces intracranial tumor burden, prolongs survival in orthotopic IDH-WT GBM mouse model, and suppresses MGMT [ Oncotarget, 2025, 16:230-248] | PubMed: 40145650 |
| KLF11/TMEM87B promoted the occurrence of glioma and decreased TMZ sensitivity [ Cell Signal, 2025, 130:111651] | PubMed: 39929351 |
| Integration of 3D bioprinting and multi-algorithm machine learning identified glioma susceptibilities and microenvironment characteristics [ Cell Discov, 2024, 10(1):39] | PubMed: 38594259 |
| Reconstructing the regulatory programs underlying the phenotypic plasticity of neural cancers [ Nat Commun, 2024, 15(1):9699] | PubMed: 39516198 |
| Integrated molecular and functional characterization of the intrinsic apoptotic machinery identifies therapeutic vulnerabilities in glioma [ Nat Commun, 2024, 15(1):10089] | PubMed: 39572533 |
| LncRNA-Mediated TPI1 and PKM2 Promote Self-Renewal and Chemoresistance in GBM [ Adv Sci (Weinh), 2024, 11(44):e2402600] | PubMed: 39342418 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
