|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C35H38Cl2N8O4 |
|||
| Molecular Weight | 705.65031 | CAS No. | 84625-61-6 | |
| Solubility (25°C)* | In vitro | DMSO | 13 mg/mL (18.42 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Itraconazole is a relatively potent inhibitor of CYP3A4 with IC50 of 6.1 nM, used as a triazole antifungal agent. Itraconazole is a potent antagonist of the Hedgehog (Hh) signaling pathway. Itraconazole suppresses the growth of glioblastoma through induction of autophagy. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Itraconazole is metabolized into hydroxy-itraconazole (OH-ITZ), a known in vivo metabolite of ITZ, and two new metabolites: keto-itraconazole (keto-ITZ) and N-desalkyl-itraconazole (ND-ITZ). Itraconazole is a substrate for CYP3A in vitro and to characterize the metabolites generated. Itraconazole exhibits an unbound Km of 3.9 nM for CYP3A. Itraconazole metabolites are as potent as or more potent CYP3A4 inhibitors than ITZ itself. [1] Itraconazole appears to act on the essential Hh pathway component Smoothened (SMO) by a mechanism distinct from that of cyclopamine and other known SMO antagonists, and prevents the ciliary accumulation of SMO normally caused by Hh stimulation. [2] Itraconazole is active against 60 clinical isolates of Aspergillus spp. with geometric mean (GM) MICs of 0.25 mg/mL. [3] Itraconazole acts primarily by impairing the synthesis of ergosterol, resulting in a defective fungal cell membrane with altered permeability and function. Itraconazole is effective for a wide variety of mycotic infections and some fungal meningeal infections. [4] Itraconazole has an affinity for mammalian cytochrome P-450 enzymes as well as for fungal P-450-dependent enzyme, and thus has the potential for clinically important interactions (e.g., astemizole, terfenadine, rifampin, oral contraceptives, H2 receptor antagonists, warfarin, cyclosporine). [5] | ||
| In vivo | Itraconazole, like other Hh pathway antagonists, can suppress Hh pathway activity and the growth of medulloblastoma in a mouse allograft model. [2] |
Protocol (from reference)
References
Customer Product Validation
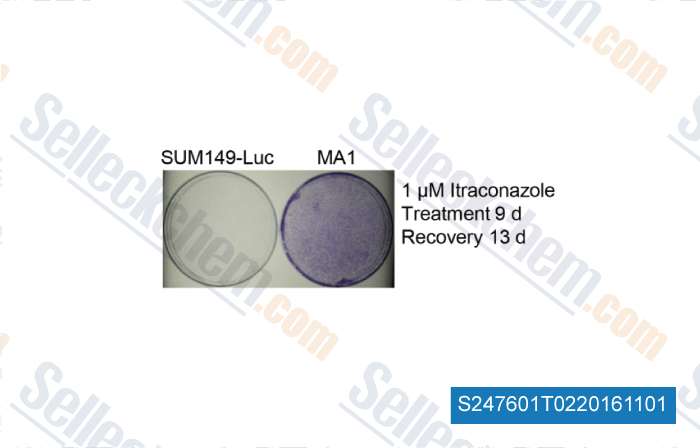
-
, , PLoS One, 2014, 9(10):e109487.
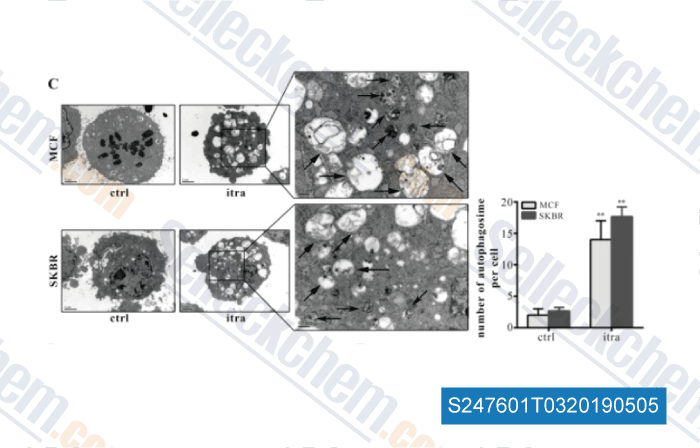
-
Data from [Data independently produced by , , Cancer Lett, 2017, 385:128-136]
Selleck's Itraconazole has been cited by 42 publications
| Improved Broad Spectrum Antifungal Drug Synergies with Cryptomycin, a Cdc50-Inspired Antifungal Peptide [ ACS Infect Dis, 2024, 10(11):3973-3993] | PubMed: 39475550 |
| Synergistic activity of the combination of falcarindiol and itraconazole in vitro against dermatophytes [ Front Cell Infect Microbiol, 2023, 13:1128000] | PubMed: 37207188 |
| Interactions between antifungals and everolimus against Cryptococcus neoformans [ Front Cell Infect Microbiol, 2023, 13:1131641] | PubMed: 37026056 |
| The Synergistic Effect of Tacrolimus (FK506) or Everolimus and Azoles Against Scedosporium and Lomentospora Species In Vivo and In Vitro [ Front Cell Infect Microbiol, 2022, 12:864912] | PubMed: 35493742 |
| Synergistic effect of pyrvinium pamoate and posaconazole against Cryptococcus neoformans in vitro and in vivo [ Front Cell Infect Microbiol, 2022, 12:1074903] | PubMed: 36569209 |
| The Antineoplastic Effect of Carboplatin Is Potentiated by Combination with Pitavastatin or Metformin in a Chemoresistant High-Grade Serous Carcinoma Cell Line [ Int J Mol Sci, 2022, 24(1)97] | PubMed: 36613537 |
| Terconazole, an Azole Antifungal Drug, Increases Cytotoxicity in Antimitotic Drug-Treated Resistant Cancer Cells with Substrate-Specific P-gp Inhibitory Activity [ Int J Mol Sci, 2022, 23(22)13809] | PubMed: 36430288 |
| An FDA-Approved Antifungal, Ketoconazole, and Its Novel Derivative Suppress tGLI1-Mediated Breast Cancer Brain Metastasis by Inhibiting the DNA-Binding Activity of Brain Metastasis-Promoting Transcription Factor tGLI1 [ Cancers (Basel), 2022, 14(17)4256] | PubMed: 36077791 |
| A Preliminary in vitro and in vivo Evaluation of the Effect and Action Mechanism of 17-AAG Combined With Azoles Against Azole-Resistant Candida spp [ Front Microbiol, 2022, 13:825745] | PubMed: 35875545 |
| Pitavastatin and Ivermectin Enhance the Efficacy of Paclitaxel in Chemoresistant High-Grade Serous Carcinoma [ Cancers (Basel), 2022, 14(18)4357] | PubMed: 36139522 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
