|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C33H38N4O6 |
|||
| Molecular Weight | 586.68 | CAS No. | 97682-44-5 | |
| Solubility (25°C)* | In vitro | DMSO | 25 mg/mL (42.61 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Irinotecan is a topoisomerase I inhibitor for LoVo cells and HT-29 cells with IC50 of 15.8 μM and 5.17 μM, respectively. | |
|---|---|---|
| Targets |
|
|
| In vitro | Irinotecan is activated to SN-38 by carboxylesterases to become able to interact with its target, topoisomerase I. Irinotecan induces similar amounts of cleavable complexes at its IC50 in LoVo cells and HT-29 cell lines. SN-38 induces a concentration-dependent formation of cleavable complexes, which is not significantly different in LoVo cells and HT-29 cell lines. Cell accumulation of Irinotecan is markedly different, reaching consistently higher levels in HT-29 cells than in LoVo cells. [1] The lactone E-ring of Irinotecan and SN-38 hydrolyses reversibly in aqueous solutions, and the interconversion between the lactone and carboxylate forms is dependent on pH and temperature. Liver is primarily responsible for the activation of Irinotecan to SN-38. At equal concentrations of Irinotecan and SN-38 glucuronide, the rate of beta-glucuronidase-mediated SN-38 production is higher than that formed from Irinotecan in both tumour and normal tissue. [2] Irinotecan is also converted to SN-38 in intestines, plasma and tumor tissues. [3] Irinotecan is significantly more active in SCLC than in NSCLC cell lines, whereas no significant difference between histological types is observed with SN-38. [4] | |
| In vivo | In COLO 320 xenografts, Irinotecan induces a maximum growth inhibition of 92%. [5] A single dose of Irinotecan significantly increases amounts of topoisomerase I covalently bound to DNA in stomach, duodenum, colon and liver. Concomitantly, the Irinotecan-treated group shows significantly higher amounts of DNA strand breaks in colon mucosa cells compared to the control group. [6] | |
| Features | Irinotecan is a prodrug that is used to treat metastatic colorectal cancer. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation

-
, , Biomaterials, 2015, 72:74-89.
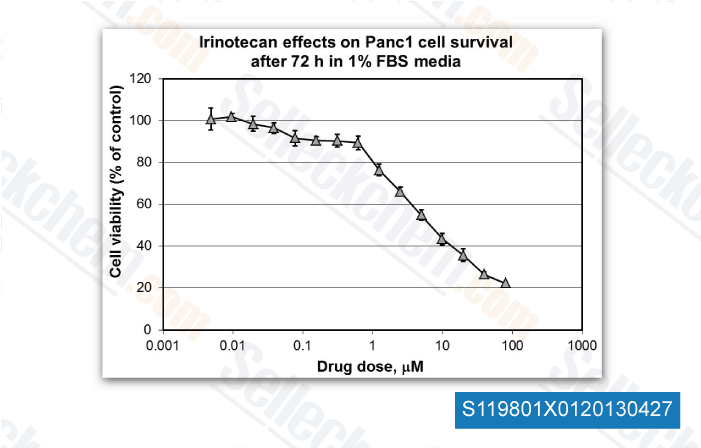
-
Data independently produced by Dr. Edita Aksamitiene from Thomas Jefferson University, , Dr. Mikhail Menshikov of Cardiology Research Center
Selleck's Irinotecan has been cited by 48 publications
| Targeting of the G9a, DNMT1 and UHRF1 epigenetic complex as an effective strategy against pancreatic ductal adenocarcinoma [ J Exp Clin Cancer Res, 2025, 44(1):13] | PubMed: 39810240 |
| AMG 193, a Clinical Stage MTA-Cooperative PRMT5 Inhibitor, Drives Antitumor Activity Preclinically and in Patients With MTAP-Deleted Cancers [ Cancer Discov, 2024, 10.1158/2159-8290.CD-24-0887] | PubMed: 39282709 |
| Developing Patient-Derived 3D-Bioprinting models of pancreatic cancer [ J Adv Res, 2024, S2090-1232(24)00413-2] | PubMed: 39278567 |
| Targeting of mutant-p53 and MYC as a novel strategy to inhibit oncogenic SPAG5 activity in triple negative breast cancer [ Cell Death Dis, 2024, 15(8):603] | PubMed: 39164278 |
| Discovery of non-genomic drivers of YAP signaling modulating the cell plasticity in CRC tumor lines [ iScience, 2024, 27(3):109247] | PubMed: 38439969 |
| Transgelin-2, a novel cancer stem cell-related biomarker, is a diagnostic and therapeutic target for biliary tract cancer [ BMC Cancer, 2024, 24(1):357] | PubMed: 38509504 |
| Visualization strategies to aid interpretation of high-dimensional genotoxicity data [ Environ Mol Mutagen, 2024, 10.1002/em.22604] | PubMed: 38757760 |
| In-situ cryo-immune engineering of tumor microenvironment with cold-responsive nanotechnology for cancer immunotherapy [ Nat Commun, 2023, 14(1):392] | PubMed: 36693842 |
| Metabolic classification suggests the GLUT1/ALDOB/G6PD axis as a therapeutic target in chemotherapy-resistant pancreatic cancer [ Cell Rep Med, 2023, 4(9):101162] | PubMed: 37597521 |
| Metabolic classification suggests the GLUT1/ALDOB/G6PD axis as a therapeutic target in chemotherapy-resistant pancreatic cancer [ Cell Rep Med, 2023, S2666-3791(23)00315-4] | PubMed: 37597521 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
