|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C33H38N4O6.HCl.3H2O |
|||
| Molecular Weight | 677.18 | CAS No. | 136572-09-3 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (147.67 mM) | |
| Ethanol | 5 mg/mL (7.38 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Irinotecan HCl Trihydrate is a hydrochloride trihydrate of irinotecan (Camptosar, Campto, CPT-11) which is a topoisomerase I inhibitor with IC50 of 15.8 and 5.17 μM for LoVo cells and HT-29 cells, respectively. | |
|---|---|---|
| Targets |
|
|
| In vitro | Irinotecan is activated to SN-38 by carboxylesterases to become able to interact with its target, topoisomerase I. Irinotecan induces similar amounts of cleavable complexes at its IC50 in LoVo cells and HT-29 cell lines. SN-38 induces a concentration-dependent formation of cleavable complexes, which is not significantly different in LoVo cells and HT-29 cell lines. Cell accumulation of Irinotecan is markedly different, reaching consistently higher levels in HT-29 cells than in LoVo cells. [1] The lactone E-ring of Irinotecan and SN-38 hydrolyses reversibly in aqueous solutions, and the interconversion between the lactone and carboxylate forms is dependent on pH and temperature. Liver is primarily responsible for the activation of Irinotecan to SN-38. At equal concentrations of Irinotecan and SN-38 glucuronide, the rate of beta-glucuronidase-mediated SN-38 production is higher than that formed from Irinotecan in both tumour and normal tissue. [2] Irinotecan is also converted to SN-38 in intestines, plasma and tumor tissues. [3] Irinotecan is significantly more active in SCLC than in NSCLC cell lines, whereas no significant difference between histological types is observed with SN-38. [4] | |
| In vivo | In COLO 320 xenografts, Irinotecan induces a maximum growth inhibition of 92%. [5] A single dose of Irinotecan significantly increases amounts of topoisomerase I covalently bound to DNA in stomach, duodenum, colon and liver. Concomitantly, the Irinotecan-treated group shows significantly higher amounts of DNA strand breaks in colon mucosa cells compared to the control group. [6] | |
| Features | Irinotecan is a prodrug that is used to treat metastatic colorectal cancer. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
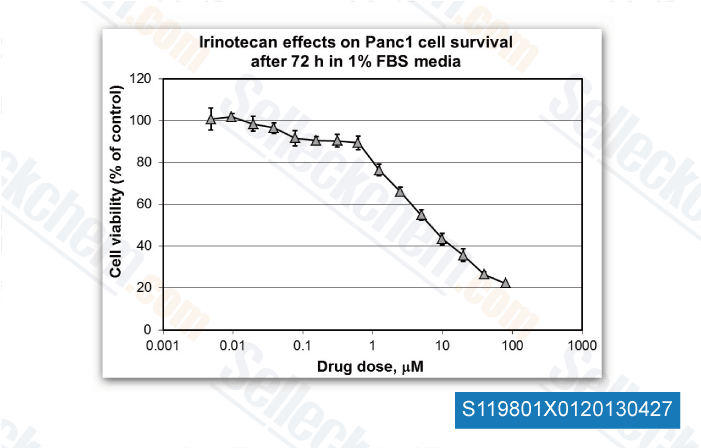
-
Data independently produced by Dr. Edita Aksamitiene from Thomas Jefferson University, , Dr. Mikhail Menshikov of Cardiology Research Center
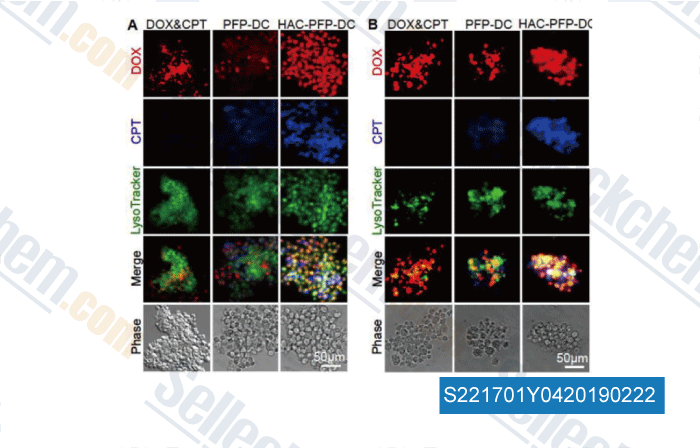
-
Data from [Data independently produced by , , Biomaterials, 2015, 72:74-89]
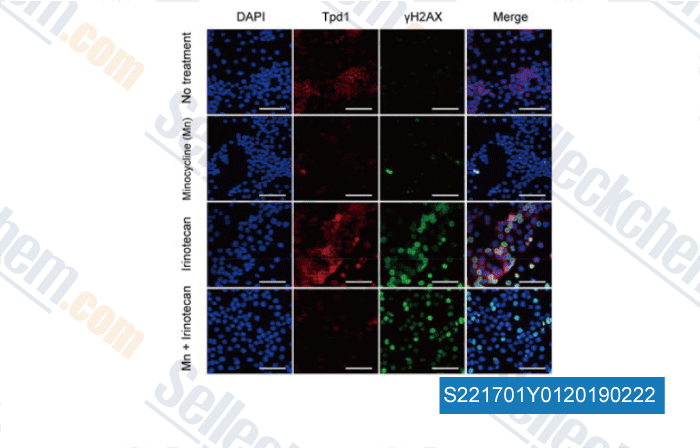
-
Data from [Data independently produced by , , Mol Cancer Ther, 2018, 17(2):508-520]
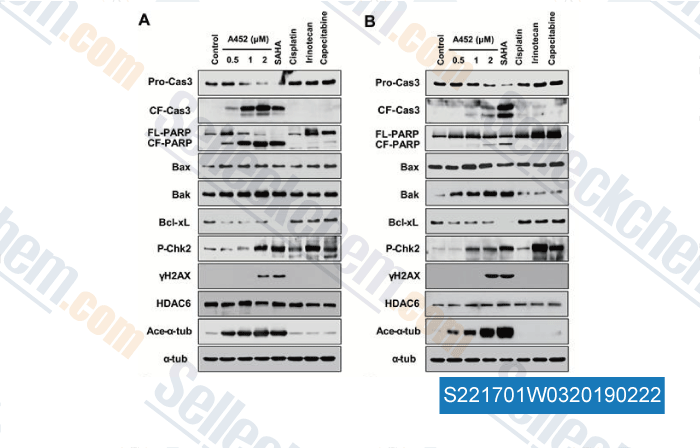
-
Data from [Data independently produced by , , Carcinogenesis, 2018, 39(1):72-83]
Selleck's Irinotecan HCl Trihydrate has been cited by 90 publications
| The novel SMYD3 inhibitor EM127 impairs DNA repair response to chemotherapy-induced DNA damage and reverses cancer chemoresistance [ J Exp Clin Cancer Res, 2024, 43(1):151] | PubMed: 38812026 |
| Establishment, characterization, and biobanking of 36 pancreatic cancer organoids: prediction of metastasis in resectable pancreatic cancer [ Cell Oncol (Dordr), 2024, 10.1007/s13402-024-00939-5] | PubMed: 38619751 |
| CHD1L Inhibitor OTI-611 Synergizes with Chemotherapy to Enhance Antitumor Efficacy and Prolong Survival in Colorectal Cancer Mouse Models [ Int J Mol Sci, 2024, 25(23)13160] | PubMed: 39684869 |
| Effects of simulated microgravity on colorectal cancer organoids growth and drug response [ Sci Rep, 2024, 14(1):25526] | PubMed: 39462078 |
| Assessment of stromal SCD-induced drug resistance of PDAC using 3D-printed zPDX model chips [ iScience, 2023, 26(1):105723] | PubMed: 36590169 |
| Artesunate ameliorates irinotecan-induced intestinal injury by suppressing cellular senescence and significantly enhances anti-tumor activity [ Int Immunopharmacol, 2023, 119:110205] | PubMed: 37104917 |
| Cutoff value of IC50 for drug sensitivity in patient-derived tumor organoids in colorectal cancer [ iScience, 2023, 26(7):107116] | PubMed: 37426352 |
| The role of pregnane X receptor (PXR-in cancer drug resistance and identification of novel PXR antagonists [ Tübingen, 2023, ] | PubMed: None |
| p53 controls choice between apoptotic and non-apoptotic death following DNA damage [ bioRxiv, 2023, 2023.01.17.524444] | PubMed: 36712034 |
| 3D imaging analysis on an organoid-based platform guides personalized treatment in pancreatic ductal adenocarcinoma [ J Clin Invest, 2022, 132(24)e151604] | PubMed: 36282600 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
