|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C26H27NO9.HCl |
|||
| Molecular Weight | 533.95 | CAS No. | 57852-57-0 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (187.28 mM) | |
| Water | 6 mg/mL (11.23 mM) | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Idarubicin HCl (4-demethoxydaunorubicin (NSC256439, 4-DMDR) HCl) is a hydrochloride salt form of Idarubicin which is an anthracycline antibiotic and a DNA topoisomerase II (topo II) inhibitor for MCF-7 cells with IC50 of 3.3 ng/mL in a cell-free assay. Idarubicin induces mTOR-dependent cytotoxic autophagy. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Idarubicin has significant cytotoxic activity against multicellular spheroids, comparable to the antiproliferative effects on monolayer cells. [1] Idarubicin inhibits CYP450 2D6.[2] Idarubicin is about 57.5-fold and 25-fold more active than doxorubicin and epirubicin, respectively. Idarubicin is able to overcome P-glycoprotein-mediated multidrug resistance. [3] Idarubicin inhibits PMN superoxide radical formation. [4] Idarubicin could be coupled to the monoclonal antibodies (anti-Ly-2.1, anti-L3T4, or anti-Thy-1) with retention of protein solubility and antibody activity. [5] Idarubicin inhibits the proliferation of NALM-6 cells with an IC50 of 12 nM. [6] |
||||
| In vivo | Reduction of Idarubicin is dependent upon ketone reductases, and proceeds more stereoselectively than that of most ketones giving rise to the (13S)-epimer almost exclusively. The high stereospecificity in Idarubicin reduction might result from chiral induction due to the presence of asymmetric centres near to the carbonyl group in Idarubicin. [7] |
||||
| Features | Idarubicin is a substrate for CYP450 2D6 and 2C9. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
Customer Product Validation
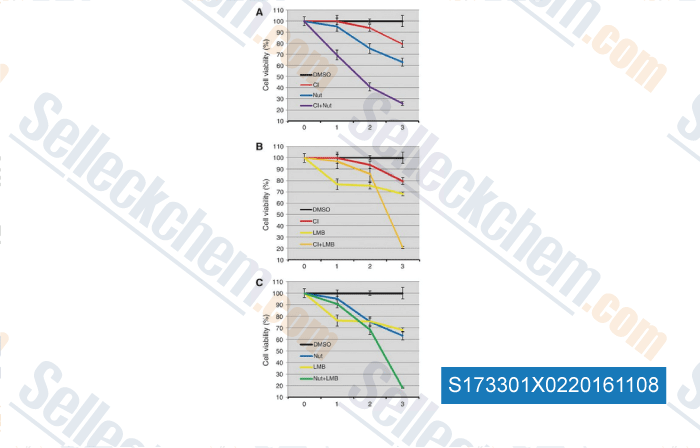
-
, , Clin Cancer Res, 2016, 22(3):746-56.
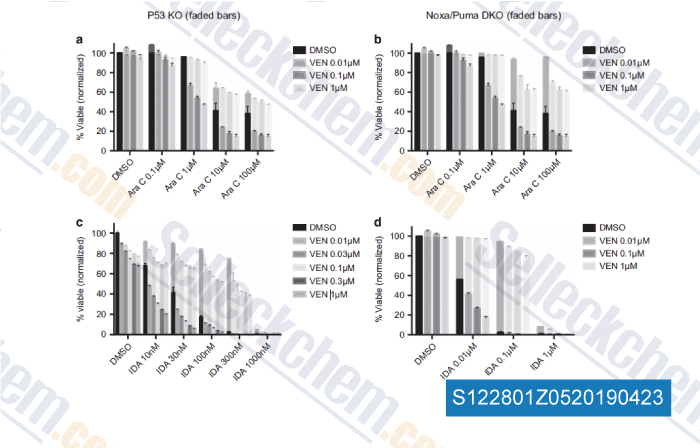
-
Data from [Data independently produced by , , Leukemia, 2018, 32(2):303-312]
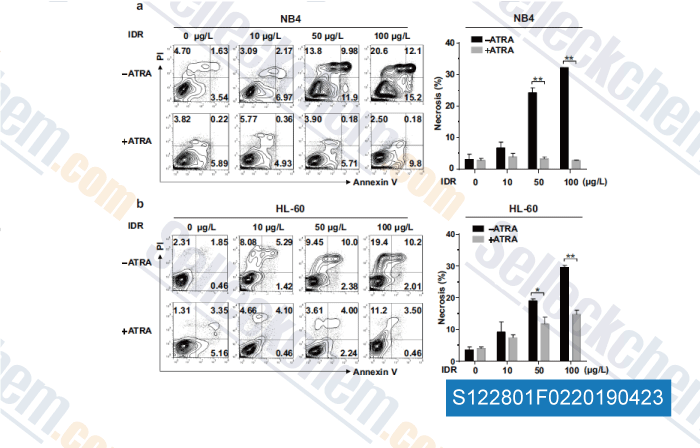
-
Data from [Data independently produced by , , Mol Cell Biochem, 2018, doi:10.1007/s11010-018-3402-0]
Selleck's Idarubicin HCl has been cited by 53 publications
| G2 arrest primes hematopoietic stem cells for megakaryopoiesis [ Cell Rep, 2024, 43(7):114388] | PubMed: 38935497 |
| Immuno-oncological effects of standard anticancer agents and commonly used concomitant drugs: an in vitro assessment [ BMC Pharmacol Toxicol, 2024, 25(1):25] | PubMed: 38444002 |
| Histone H3 lysine 27 crotonylation mediates gene transcriptional repression in chromatin [ Mol Cell, 2023, 83(13):2206-2221.e11] | PubMed: 37311463 |
| Histone H3 lysine 27 crotonylation mediates gene transcriptional repression in chromatin [ Mol Cell, 2023, 83(13):2206-2221.e11] | PubMed: 37311463 |
| Spermatogenesis associated serine rich 2 like plays a prognostic factor and therapeutic target in acute myeloid leukemia by regulating the JAK2/STAT3/STAT5 axis [ J Transl Med, 2023, 21(1):115] | PubMed: 36774517 |
| Relevance of the organic anion transporting polypeptide 1B3 (OATP1B3) in the personalized pharmacological treatment of hepatocellular carcinoma [ Biochem Pharmacol, 2023, 214:115681] | PubMed: 37429423 |
| p53 controls choice between apoptotic and non-apoptotic death following DNA damage [ bioRxiv, 2023, 2023.01.17.524444] | PubMed: 36712034 |
| Acquired mutations in BAX confer resistance to BH3-mimetic therapy in Acute Myeloid Leukemia [ Blood, 2022, blood.2022016090] | PubMed: 36219880 |
| Establishment and large-scale validation of a three-dimensional tumor model on an array chip for anticancer drug evaluation [ Front Pharmacol, 2022, 13:1032975] | PubMed: 36313330 |
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
