|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C17H18N6 |
|||
| Molecular Weight | 306.37 | CAS No. | 941678-49-5 | |
| Solubility (25°C)* | In vitro | DMSO | 61 mg/mL (199.1 mM) | |
| Ethanol | 61 mg/mL (199.1 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Ruxolitinib is the first potent, selective, JAK1/2 inhibitor to enter the clinic with IC50 of 3.3 nM/2.8 nM in cell-free assays, >130-fold selectivity for JAK1/2 versus JAK3. Ruxolitinib kills tumor cells through toxic mitophagy. Ruxolitinib induces autophagy and enhances apoptosis. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | INCB018424 potently and selectively inhibits JAK2V617F-mediated signaling and proliferation in Ba/F3 cells and HEL cells. INCB018424 markedly increases apoptosis in a dose dependent manner in Ba/F3 cells. INCB018424 (64 nM) results in a doubling of cells with depolarized mitochondria in Ba/F3 cells. INCB018424 inhibits proliferating of erythroid progenitors from normal donors and polycythemia vera patients with IC50 of 407 nM and 223 nM, respectively. INCB018424 demonstrates remarkable potency against erythroid colony formation with IC50 of 67nM. [1] | ||||
| In vivo | INCB018424 (180 mg/kg, orally, twice a day) results in survive rate of greater than 90% by day 22 in a JAK2V617F-driven mouse model. INCB018424 (180 mg/kg, orally, twice a day) markedly reduces splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminated neoplastic cells, resulting in significantly prolonged survival without myelosuppressive or immunosuppressive effects in a JAK2V617F-driven mouse model. [1] The primary end point is reached in 41.9% of patients in the Ruxolitinib group as compared with 0.7% in the placebo group in the double-blind trial of myelofibrosis. Ruxolitinib results in maintaining of reduction in spleen volume and improvement of 50% or more in the total symptom score. [2] A total of 28% of the patients in the Ruxolitinib (15 mg twice daily) group has at least a 35% reduction in spleen volume at week 48 in patients with myelofibrosis, as compared with 0% in the group receiving the best available therapy. The mean palpable spleen length has decreased by 56% with Ruxolitinib but has increased by 4% with the best available therapy at week 48. Patients in the ruxolitinib group has an improvement in overall quality-of-life measures and a reduction in symptoms associated with myelofibrosis. [3] |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[1] |
|
| Animal Study:[1] |
|
References
|
Customer Product Validation
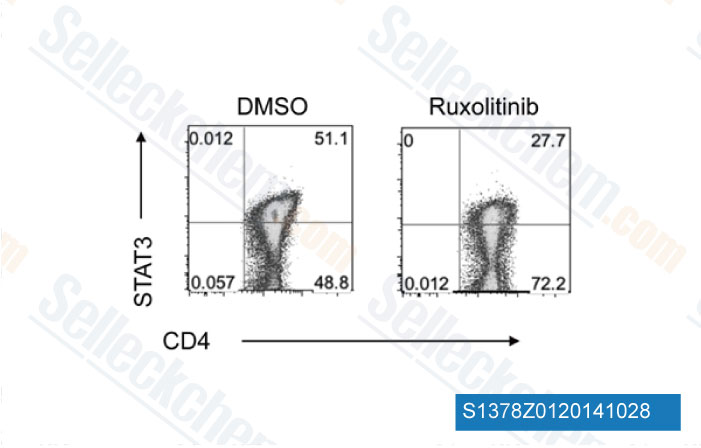
-
Data from [Data independently produced by Blood, 2014, 123(24), 3832-42]
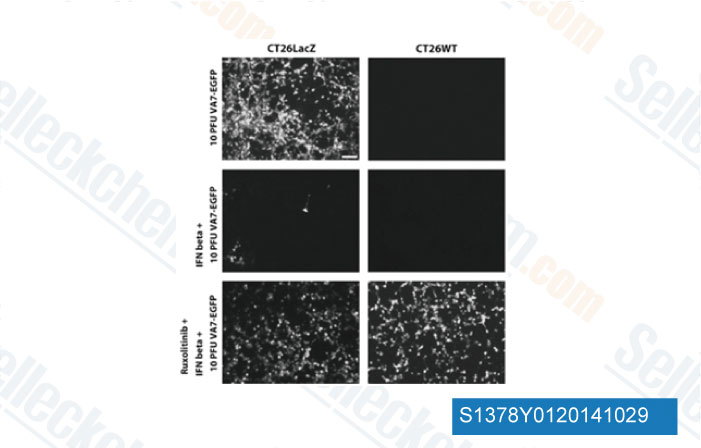
-
Data from [Data independently produced by Gene Ther, 2014, 10.1038/gt.2014.83]
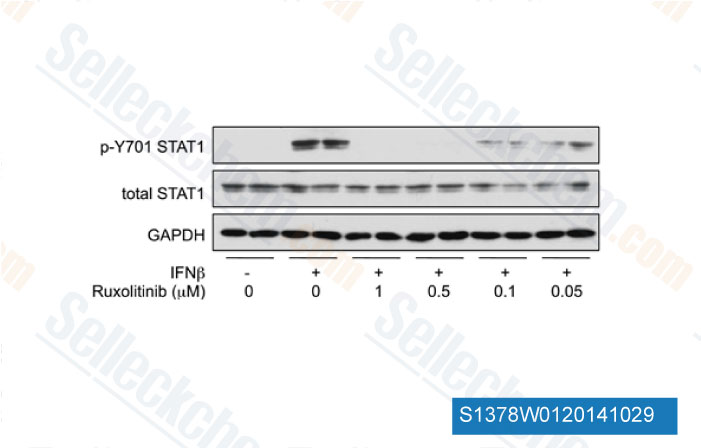
-
Data from [Data independently produced by J Immunol, 2012, 189(6), 2784-92]
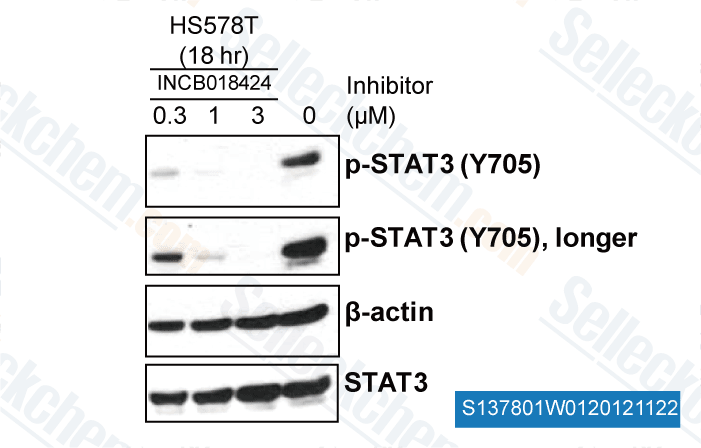
-
, , Yong Weon Yi Georgetown University
Selleck's Ruxolitinib has been cited by 671 publications
| RSK1 is an exploitable dependency in myeloproliferative neoplasms and secondary acute myeloid leukemia [ Nat Commun, 2025, 16(1):492] | PubMed: 39820365 |
| The balance between IFN-γ and ERK/MAPK signaling activities ensures lifelong maintenance of intestinal stem cells [ Cell Rep, 2025, S2211-1247(25)00057-9] | PubMed: 39952238 |
| IFN-γ licenses normal and pathogenic ALPK1/TIFA pathway in human monocytes [ iScience, 2025, 28(1):111563] | PubMed: 39868044 |
| Selective JAK2 pathway inhibition enhances anti-leukemic functionality in CD19 CAR-T cells [ Cancer Immunol Immunother, 2025, 74(3):79] | PubMed: 39891728 |
| Ruxolitinib as a novel therapeutic agent targeting mitochondrial function and chemo-resistance in nasopharyngeal carcinoma [ Biochem Biophys Res Commun, 2025, 753:151486] | PubMed: 39965265 |
| Noncanonical role of Golgi-associated macrophage TAZ in chronic inflammation and tumorigenesis [ Sci Adv, 2025, 11(4):eadq2395] | PubMed: 39841821 |
| Combined KRAS-MAPK pathway inhibitors and HER2-directed drug conjugate is efficacious in pancreatic cancer [ Nat Commun, 2024, 15(1):2503] | PubMed: 38509064 |
| Inhibition of neutrophil swarming by type I interferon promotes intracellular bacterial evasion [ Nat Commun, 2024, 15(1):8663] | PubMed: 39375351 |
| Gadd45g insufficiency drives the pathogenesis of myeloproliferative neoplasms [ Nat Commun, 2024, 15(1):2989] | PubMed: 38582902 |
| Defective N-glycosylation of IL6 induces metastasis and tyrosine kinase inhibitor resistance in lung cancer [ Nat Commun, 2024, 15(1):7885] | PubMed: 39251588 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
