|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C42H62O16 |
||||||||||
| Molecular Weight | 822.93 | CAS No. | 1405-86-3 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (121.51 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Glycyrrhizin (NSC 167409, Glycyrrhizic Acid) is a direct HMGB1(high mobility group box 1) inhibitor that inhibits HMGB1-dependent inflammatory molecule expression and oxidative stress; modulates P38 and P-JNK but not p-ERK signalling; Also inhibits 11 beta-hydroxysteroid dehydrogenase and monoamine oxidase (MAO). | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
||||||
| In vitro | Glycyrrhizin inhibits monoamine oxidase (MAO) with the IC50 value of 0.16 μM[4]. |
||||||
| In vivo | Glycyrrhizin increases the levels of monoamines like epinephrine and dopamine in brains of mice[3]. |
Protocol (from reference)
| Animal Study: |
|
|---|
References
Customer Product Validation
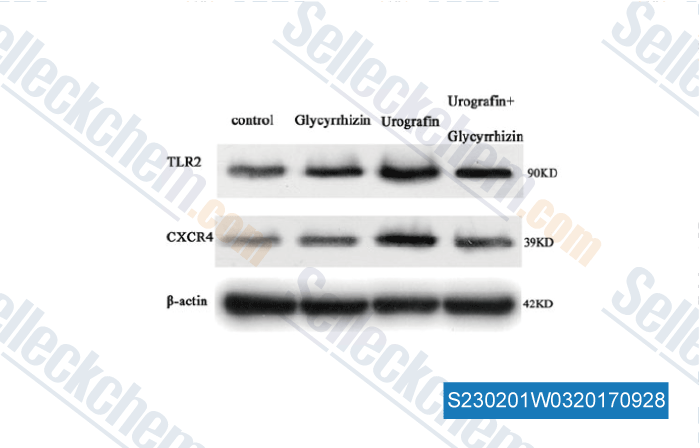
-
Data from [Data independently produced by , , DNA Cell Biol, 2017, 36(1):67-76]
Selleck's Glycyrrhizin (NSC 167409) has been cited by 42 publications
| A skin organoid-based infection platform identifies an inhibitor specific for HFMD [ Nat Commun, 2025, 16(1):2513] | PubMed: 40082449 |
| EZH2/miR-142-3p/HMGB1 axis mediates chondrocyte pyroptosis by regulating endoplasmic reticulum stress in knee osteoarthritis [ Chin Med J (Engl), 2025, 138(1):79-92] | PubMed: 39704001 |
| Cytosolic Hmgb1 accumulation in mesangial cells aggravates diabetic kidney disease progression via NFκB signaling pathway [ Cell Mol Life Sci, 2024, 81(1):408] | PubMed: 39287634 |
| Targeting acetylated high mobility group box 1 protein (HMGB1) and toll-like receptor (TLR4) interaction to alleviate hypertension and neuroinflammation in fructose-fed rats [ Br J Pharmacol, 2024, 10.1111/bph.17402] | PubMed: 39627019 |
| Hypermethylation of PPARG-encoding gene promoter mediates fine particulate matter-induced pulmonary fibrosis by regulating the HMGB1/NLRP3 axis [ Ecotoxicol Environ Saf, 2024, 272:116068] | PubMed: 38330871 |
| HMGB1-Mediated Cell Death-A Crucial Element in Post-Hepatectomy Liver Failure [ Int J Mol Sci, 2024, 25(13)7150] | PubMed: 39000266 |
| Glycyrrhizin ameliorates colorectal cancer progression by regulating NHEJ pathway through inhibiting HMGB1-induced DNA damage response [ Sci Rep, 2024, 14(1):24948] | PubMed: 39438689 |
| NETs exacerbate placental inflammation and injury through high mobility group protein B1 during preeclampsia [ Placenta, 2024, 159:131-139] | PubMed: 39718052 |
| Glycyrrhizin inhibits LPS-induced neutrophil-like release of NETs [ Am J Transl Res, 2024, 16(10):5507-5515] | PubMed: 39544807 |
| The regulation and function of acetylated high-mobility group box 1 during implantation and decidualization [ Front Immunol, 2023, 14:1024706] | PubMed: 36761729 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
