|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C11H11F3N2O3 |
|||
| Molecular Weight | 276.21 | CAS No. | 13311-84-7 | |
| Solubility (25°C)* | In vitro | DMSO | 55 mg/mL (199.12 mM) | |
| Ethanol | 55 mg/mL (199.12 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Flutamide (SCH-13521) is an antiandrogen drug, with its active metablolite binding at androgen receptor with Ki values of 55 nM, and primarily used to treat prostate cancer. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Flutamide (Eulexin) is an antiandrogen drug. Flutamide-OH, the active metabolite of flutamide, directly binds at rat anterior pituitary androgen receptor with Ki values of 55 nM. [1] Flutamide does not affect the proliferation of an androgen-sensitive clone of the mouse mammary carcinoma Shionogi SC-l 15 cells in culture, shows only antiandrogenic effect, but not androgenic effect. [2] Flutamide provides treatment for prostate cancer when used along with leuprolide. [3] | ||
| In vivo | Flutamide causes a markedly reduction in rat ventral prostate weight from 319 mg to 245 mg. A combination of flutamide and LHRH agonist induces an additive effect with a decrease in prostate weight to 101 mg, and an marked drop in prostatic ODC activity. [4] |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[2] |
|
| Animal Study:[4] |
|
References
Customer Product Validation
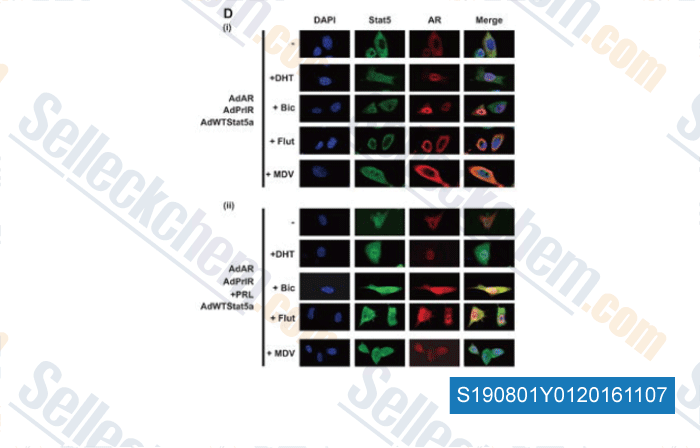
-
, , Mol Cancer Ther, 2015, 14(3):713-26.
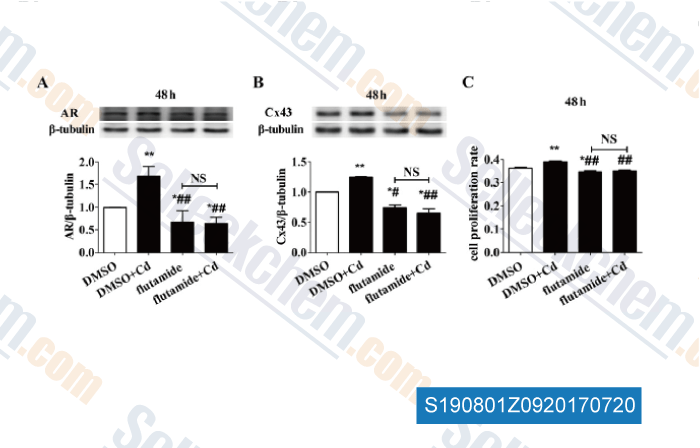
-
Data from [Data independently produced by , , J Appl Toxicol, 2017, 37(8):933-942]
Selleck's Flutamide has been cited by 19 publications
| Androgen signaling inhibits de novo lipogenesis to alleviate lipid deposition in zebrafish [ Zool Res, 2024, 45(2):355-366] | PubMed: 38485505 |
| Sex hormone receptors, calcium-binding protein and Yap1 signaling regulate sex-dependent liver cell proliferation following partial hepatectomy [ Dis Model Mech, 2024, 17(10)dmm050900] | PubMed: 39397390 |
| Forsythiaside A Improves the Inhibitory Efficiency of Recombinant Protein Vaccines against Bovine Viral Diarrhea Virus Infection [ Int J Mol Sci, 2022, 23-169390] | PubMed: 36012654 |
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
| Establishment and characterization of NCC-UPS4-C1: a novel cell line of undifferentiated pleomorphic sarcoma from a patient with Li-Fraumeni syndrome [ Hum Cell, 2022, 10.1007/s13577-022-00671-y] | PubMed: 35118583 |
| Quantitative chemoproteomics reveals O-GlcNAcylation of cystathionine γ-lyase (CSE) represses trophoblast syncytialization [ Cell Chem Biol, 2021, 28(6):788-801.e5] | PubMed: 33626323 |
| Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00639-4] | PubMed: 34731453 |
| Establishment and characterization of NCC-MFS4-C1: a novel patient-derived cell line of myxofibrosarcoma [ Hum Cell, 2021, 34(6):1911-1918] | PubMed: 34383271 |
| Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00579-z] | PubMed: 34304386 |
| Establishment and characterization of patient-derived cancer models of malignant peripheral nerve sheath tumors. [ Cancer Cell Int, 2020, 19;20:58] | PubMed: 32099531 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
