|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C28H27NO4S.HCl |
||||||
| Molecular Weight | 510.04 | CAS No. | 82640-04-8 | ||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (196.06 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Raloxifene (LY156758, Keoxifene) HCl is a selective and orally active estrogen receptor modulator (SERM), which inhibits human cytosolic aldehyde oxidase-catalyzed phthalazine oxidation activity with IC50 of 5.7 nM. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Raloxifene, has been demonstrated as a potent uncompetitive inhibitor of human liver aldehyde oxidase-catalyzed oxidation of phthalazine, vanillin, and nicotine-Delta1'(5')-iminium ion, with Ki values of 0.87 nM, 1.2 nM and 1.4 nM. Raloxifene has also been shown to be a noncompetitive inhibitor of an aldehyde oxidase-catalyzed reduction reaction of a hydroxamic acid-containing compound, with a Ki of 51 nM. [1] Raloxifene activates TGF beta 3 promoter as a full agonist at nanomolar concentrations, and raloxifene inhibits the estrogen response element-containing vitellogenin promoter expression as a pure estrogen antagonist in transient transfection assays. [2] |
||
| In vivo | Raloxifene restores both bone mineral density and TGF beta 3 messenger RNA expression in the femur to levels measured in intact rats. [2] Raloxifene (0.1 mg/kg-10 mg/kg, orally for 5 weeks) increases bone mineral density in the distal femur and proximal tibia in ovariectomized (OVX) rat. Raloxifene reduces serum cholesteroloral with ED50 of 0.2 mg/kg in ovariectomized (OVX) rat. Raloxifene diverges dramatically from estrogen in its lack of significant estrogenic effects on uterine tissue. [3] Raloxifene prevents cancellous osteopenia as well as the changes in radial bone growth, bone resorption, and blood cholesterol, but is less effective in reducing cancellous bone formation and does not prevent uterine atrophy in ovariectomized (OVX) rats. [4] Raloxifene (3 mg/kg/day) has potent estrogenic activity on bone resorption and serum cholesterol, a lesser effect on bone formation, and minimal activity on uterine wet weight in ovariectomized (OVX) rats. [5] |
||
| Features | Raloxifene is as effective as tamoxifen in reducing the incidence of breast cancer in postmenopausal women at increased risk. |
Protocol (from reference)
| Animal Study: |
|
|---|
References
Customer Product Validation
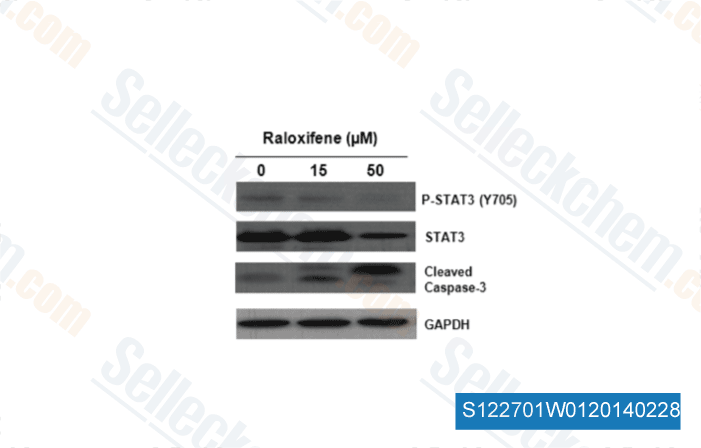
-
Data from [J Med Chem, 2014, 57(3), 632-41]
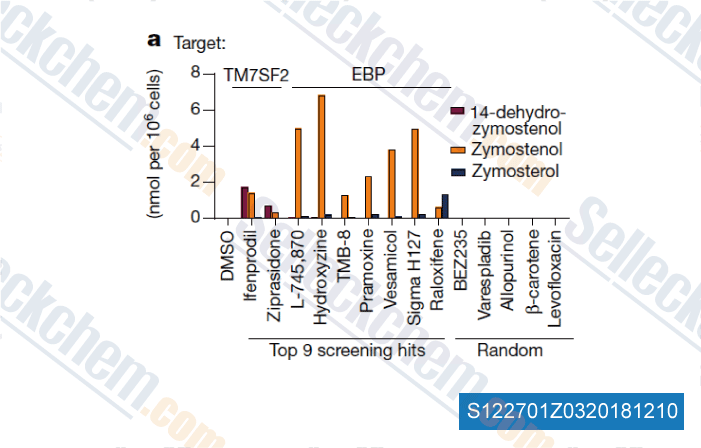
-
Data from [Data independently produced by , , Nature, 2018, 560(7718):372-376]
Selleck's Raloxifene HCl has been cited by 13 publications
| Antiviral Activity of Selective Estrogen Receptor Modulators against Severe Fever with Thrombocytopenia Syndrome Virus In Vitro and In Vivo [ Viruses, 2024, 16(8)1332] | PubMed: 39205306 |
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
| Establishment and characterization of NCC-UPS4-C1: a novel cell line of undifferentiated pleomorphic sarcoma from a patient with Li-Fraumeni syndrome [ Hum Cell, 2022, 10.1007/s13577-022-00671-y] | PubMed: 35118583 |
| A compendium of kinetic modulatory profiles identifies ferroptosis regulators [ Nat Chem Biol, 2021, 10.1038/s41589-021-00751-4] | PubMed: 33686292 |
| Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00639-4] | PubMed: 34731453 |
| Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00579-z] | PubMed: 34304386 |
| Establishment and characterization of NCC-MFS4-C1: a novel patient-derived cell line of myxofibrosarcoma [ Hum Cell, 2021, 34(6):1911-1918] | PubMed: 34383271 |
| Identification of Estrogen Receptor Modulators as Inhibitors of Flavivirus Infection [ Antimicrob Agents Chemother, 2020, 1;AAC.00289-20] | PubMed: 32482672 |
| Estrogen-Related Hormones Induce Apoptosis by Stabilizing Schlafen-12 Protein Turnover. [ Mol Cell, 2019, 75(6):1103-1116.e9] | PubMed: 31420216 |
| Controlling distinct signaling states in cultured cancer cells provides a new platform for drug discovery [ FASEB J, 2019, 10.1096/fj.201802603RR] | PubMed: 31145643 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
