|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C8H14N2O4Pt |
|||
| Molecular Weight | 397.29 | CAS No. | 61825-94-3 | |
| Solubility (25°C)* | In vitro | Water | 2 mg/mL (5.03 mM) | |
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Oxaliplatin is a DNA alkylating agent that activates autophagy. Oxaliplatin inhibits DNA synthesis by conforming DNA adducts in RT4, TCCSUP, A2780, HT-29, U-373MG, U-87MG, SK-MEL-2, and HT-144 cells.Solutions are unstable and should be fresh-prepared.DMSO is not recommended to dissolve platinum-based drugs, which can easily lead to drug inactivation. | |
|---|---|---|
| Targets |
|
|
| In vitro | The main mechanism of action of Oxaliplatin is mediated through the formation of DNA–adducts. Oxaliplatin induces primary and secondary DNA lesions that lead to cell apoptosis. [1] Oxaliplatin is active against human melanoma cell lines C32 and G361 with IC50 of 0.98 mM and 0.14 mM, respectively. [2] Oxaliplatin effectively inhibits bladder carcinoma cell lines RT4 and TCCSUP, ovarian carcinoma cell line A2780, colon carcinoma cell line HT-29, glioblastoma cell lines U-373MG and U-87MG, and melanoma cell lines SK-MEL-2 and HT-144 with IC50 of 11 μM, 15 μM, 0.17 μM, 0.97 μM, 2.95 μM, 17.6 μM, 30.9 μM and 7.85 μM, respectively. [4] |
|
| In vivo | A weekly i.p. injection of Oxaliplatin at 10 mg/kg to nude mice bearing hepatocellular HCCLM3 tumors significantly reduces tumor volume and apoptotic index. [6] Oxaliplatin (5mg/kg, i.v. on days 1, 5 and 9) is active on T-leukemia-lymphoma L40 AKR with T/C of 1.77. Oxaliplatin is also efficient on intracerebrally grafted L1210 leukemia, MA 16-C xenografts, B16 melanoma xenografts, Lewis lung xenografts and C26 colon carcinoma xenografts. [7] Oxaliplatin induces impairment of retrograde neuronal transport in mice. [8] |
|
| Features | This product is not recommended to be dissolved in dimethylsulfoxide (DMSO).[9] |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
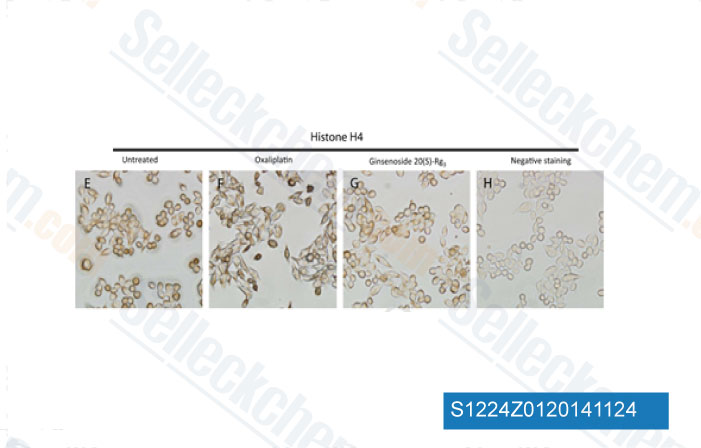
-
Data from [J Proteomics, 2014, 10.1016/j.jprot.2014.10.009]
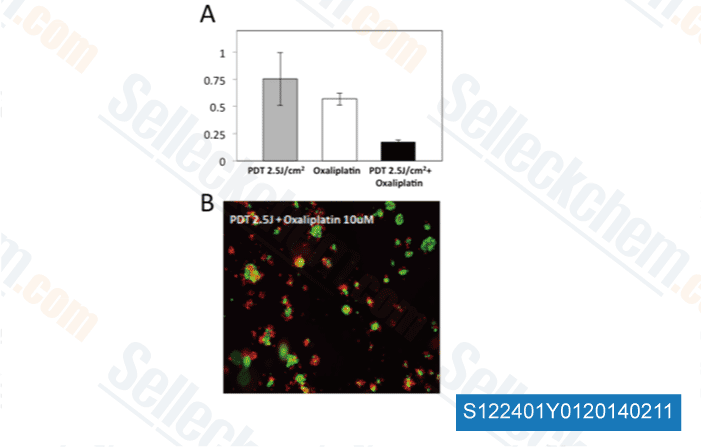
-
Data from [Optical Methods for Tumor Treatment and Detection, 2013, 10.1117/12.2010730]
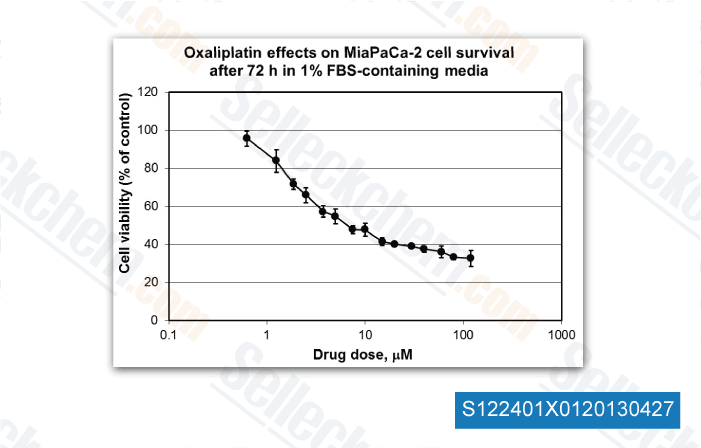
-
, 2013, Dr. Edita Aksamitiene from Thomas Jefferson University
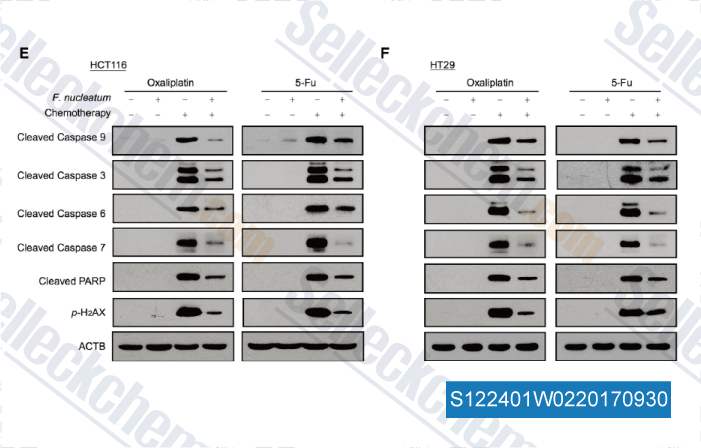
-
Data from [Data independently produced by , , Cell, 2017, 170(3):548-563.e16]
Selleck's Oxaliplatin has been cited by 265 publications
| Prediction of Patient Drug Response via 3D Bioprinted Gastric Cancer Model Utilized Patient-Derived Tissue Laden Tissue-Specific Bioink [ Adv Sci (Weinh), 2025, 12(10):e2411769] | PubMed: 39748450 |
| Targeting of the G9a, DNMT1 and UHRF1 epigenetic complex as an effective strategy against pancreatic ductal adenocarcinoma [ J Exp Clin Cancer Res, 2025, 44(1):13] | PubMed: 39810240 |
| USP1 promotes pancreatic cancer progression and autophagy by deubiquitinating ATG14 [ J Biol Chem, 2025, 301(3):108190] | PubMed: 39814232 |
| SRT3025-loaded cell membrane hybrid liposomes (3025@ML) enhanced anti-tumor activity of Oxaliplatin via inhibiting pyruvate kinase M2 and fatty acid synthase [ Lipids Health Dis, 2025, 24(1):14] | PubMed: 39825408 |
| NAD+/SIRT1 pathway regulates glycolysis to promote oxaliplatin resistance in colorectal cancer [ World J Gastroenterol, 2025, 31(11):100785] | PubMed: 40124268 |
| DDX18 influences chemotherapy sensitivity in colorectal cancer by regulating genomic stability [ Exp Cell Res, 2025, 444(1):114344] | PubMed: 39577603 |
| Eribulin Induction of Immunogenic Cell Death (ICD): Comparison With Other Cytotoxic Agents and Temporal Relationship of ICD Biomarkers [ Anticancer Res, 2025, 45(1):39-53] | PubMed: 39740820 |
| A novel DNA repair protein, N-Myc downstream regulated gene 1 (NDRG1), links stromal tumour microenvironment to chemoresistance [ bioRxiv, 2025, 2025.01.22.634323] | PubMed: 39896456 |
| YTHDF2 in peritumoral hepatocytes mediates chemotherapy-induced antitumor immune responses through CX3CL1-mediated CD8+ T cell recruitment [ Mol Cancer, 2024, 23(1):186] | PubMed: 39237909 |
| LINC01852 inhibits the tumorigenesis and chemoresistance in colorectal cancer by suppressing SRSF5-mediated alternative splicing of PKM [ Mol Cancer, 2024, 23(1):23] | PubMed: 38263157 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
