|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C11H14ClNO3 |
||||||
| Molecular Weight | 243.69 | CAS No. | 99873-43-5 | ||||
| Solubility (25°C)* | In vitro | DMSO | 49 mg/mL (201.07 mM) | ||||
| Ethanol | 49 mg/mL (201.07 mM) | ||||||
| Water | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Droxinostat (NS 41080) is a selective inhibitor of HDAC, mostly for HDACs 6 and 8 with IC50 of 2.47 μM and 1.46 μM, greater than 8-fold selective against HDAC3 and no inhibition to HDAC1, 2, 4, 5, 7, 9, and 10. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
|||||||||||
| In vitro | Droxinostat is originally identified as a sensitizer of PPC-1 cells to FAS and TRAIL by downregulating the expression of c-Fas-associated death domain-like interleukin-1-converting enzyme-like inhibitory protein (c-FLIP). [1] In PPC-1 cells cultured in suspension but not adherent conditions, Droxinostat (20 μM–60 μM) sensitizes cells to anoikis by initially activating caspase 8 with subsequent activation of the mitochondrial pathway. Similarly, Droxinostat also sensitizes other cancer cell lines including PC-3, DU-145, T47D, and OVCAR-3, but not LNCaP or MB-MDA-468, to anoikis or CH-11-induced apoptosis. [2] However, the direct targets of Droxinostat remains enigma until recently. It is revealed that in histone deacetylases (HDAC) isoform 1-10, Droxinostat selective inhibits HDAC3, 6, and 8, with IC50 values of 16.9 μM, 2.47 μM, and 1.46 μM, respectively, without inhibiting other HDAC members (IC50 > 20 μM). [3] In MCF-7 breast cancer cells, Droxinostat (10 μM–100 μM) sensitizes cells to apoptosis by decreasing c-FLIPL and c-FLIPS expression, reducing cell survival, and inducing apoptosis. [4] | |||||||||||
| In vivo | In SCID mice models, Droxinostat (30 μM)-treated PPC-1 cells results in decreased distant tumor formation than untreated cells. [2] | |||||||||||
| Features | Selective inhibitor of HDAC3, HDAC6,and HDAC8. |
Protocol (from reference)
| Kinase Assay:[3] |
|
|---|---|
| Cell Assay:[1] |
|
References
Customer Product Validation
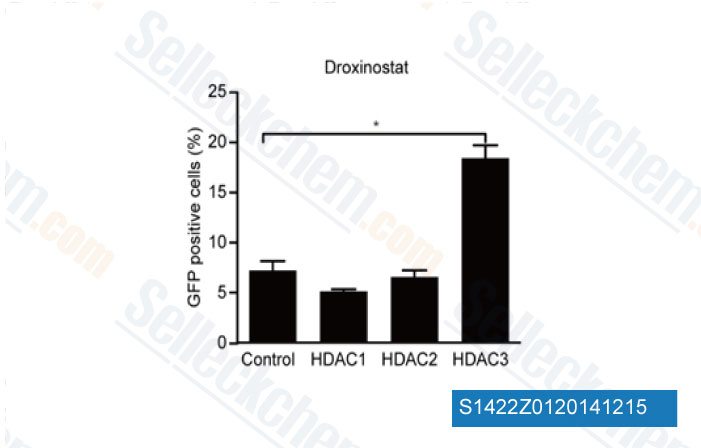
-
Data from [Data independently produced by PLoS One, 2014, 9(8), e102684]
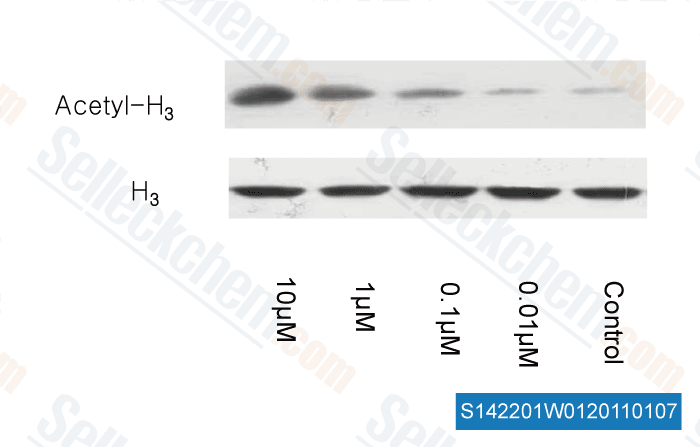
-
, , Dr. Zhang of Tianjin Medical University
Selleck's Droxinostat has been cited by 14 publications
| Afatinib or Bevacizumab in combination with Osimertinib efficiently control tumor development in orthotopic murine models of non-small lung cancer [ PLoS One, 2024, 19(6):e0304914] | PubMed: 38935790 |
| Effects of histone H4 hyperacetylation on inhibiting MMP2 and MMP9 in human amniotic epithelial cells and in premature rupture of fetal membranes [ Exp Ther Med, 2021, 21(5):515] | PubMed: 33815588 |
| Glycogen synthase kinase-3 inhibition overcomes epithelial-mesenchymal transition-associated resistance to osimertinib in EGFR-mutant lung cancer [ Cancer Sci, 2020, 111(7):2374-2384] | PubMed: 32391602 |
| Epigenetic Reprogramming with Antisense Oligonucleotides Enhances the Effectiveness of Androgen Receptor Inhibition in Castration-Resistant Prostate Cancer [ Cancer Res, 2018, 78(20):5731-5740] | PubMed: 30135193 |
| Histone Deacetylase 3 Inhibition Overcomes BIM Deletion Polymorphism–Mediated Osimertinib Resistance in EGFR-Mutant Lung Cancer [Tanimoto A Clin Cancer Res, 2017, 23(12):3139-3149] | PubMed: 27986747 |
| Identification of a cancer stem cell-specific function for the histone deacetylases, HDAC1 and HDAC7, in breast and ovarian cancer. [ Oncogene, 2017, 36(12):1707-1720] | PubMed: 27694895 |
| Inhibition of class I histone deacetylases by romidepsin potently induces Epstein-Barr virus lytic cycle and mediates enhanced cell death with ganciclovir [Hui KF, et al. Int J Cancer, 2016, 138(1):125-36] | PubMed: 26205347 |
| Droxinostat, a Histone Deacetylase Inhibitor, Induces Apoptosis in Hepatocellular Carcinoma Cell Lines via Activation of the Mitochondrial Pathway and Downregulation of FLIP. [ Transl Oncol, 2016, 9(1):70-78] | PubMed: 26947884 |
| AF4 and AF4-MLL mediate transcriptional elongation of 5-lipoxygenase mRNA by 1, 25-dihydroxyvitamin D3 [ Oncotarget, 2015, 6(28):25784-800] | PubMed: 26329759 |
| PU.1 Suppresses Th2 Cytokine Expression via Silencing of GATA3 Transcription in Dendritic Cells [ PLoS One, 2015, 10(9):e0137699] | PubMed: 26361334 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
