|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C10H11ClFN5O3 |
|||
| Molecular Weight | 303.68 | CAS No. | 123318-82-1 | |
| Solubility (25°C)* | In vitro | DMSO | 60 mg/mL (197.57 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Clofarabine (Clolar) inhibits the enzymatic activities of ribonucleotide reductase (RNR) (IC50 = 65 nM) and DNA polymerase. Clofarabine induces autophagy and apoptosis. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Clofarabine is efficiently transported into cells via two facilitative or equilibrative nucleoside transporters, hENT1 and hENT2, and a concentrative nucleoside transporter, hCNT253. Clofarabine is phosphorylated in a stepwise manner by cytosolic kinases to the nucleotide analogues clofarabine 5′-mono-, di- and triphosphate following entry into cells, with Clofarabine triphosphate being the active form. Clofarabine 5′-mono-, di- and triphosphate are not substrates for nucleoside transporters and must be enzymatically converted by 5′-nucleotidase back to their dephosphorylated nucleoside form for transport out of the cell. Clofarabine triphosphate is a potent inhibitor of ribonucleotide reductase (IC50 = 65 nM), presumably by binding to the allosteric site on the regulatory subunit. Clofarabine has also been shown to act directly on mitochondria by altering the transmembrane potential with release of cytochrome c, apoptotic-inducing factor (AIF), apoptosis protease-activating factor 1 (APAF1) and caspase 9 into the cytosol. Clofarabine demonstrates strong in vitro growth inhibition and cytotoxic activity (IC50 values = 0.028–0.29 μM) in a wide variety of leukaemia and solid tumour cell lines. Clofarabine has been shown to increase the activity of dCK in HL60 cells, and increases the formation of the mono-, di-, and triphosphates of ara-C in K562 cells36. [1] Clofarabine (10 μM) inhibits the repair initiated by 4-hydroperoxycyclophosphamide (4-HC), with inhibition peaking at the intracellular concentrations of 5 μM in chronic lymphocytic leukemia (CLL) lymphocytes. Clofarabine (10 μM) combined with 4-hydroperoxycyclophosphamide (4-HC) produces more than additive apoptotic cell death than the sum of each alone. [2] Clofarabine (1 μM) combined with ara-C (10 μM) results in a biochemical modulation of ara-CTP and synergistic cell kill in K562 cells. [3] | ||
| In vivo | Clofarabine administered intraperitoneally has significant activity against a wide variety of human tumour xenografts implanted subcutaneously in athymic nude or severe combined immune deficiency mice. [1] |
Protocol (from reference)
References
|
Customer Product Validation
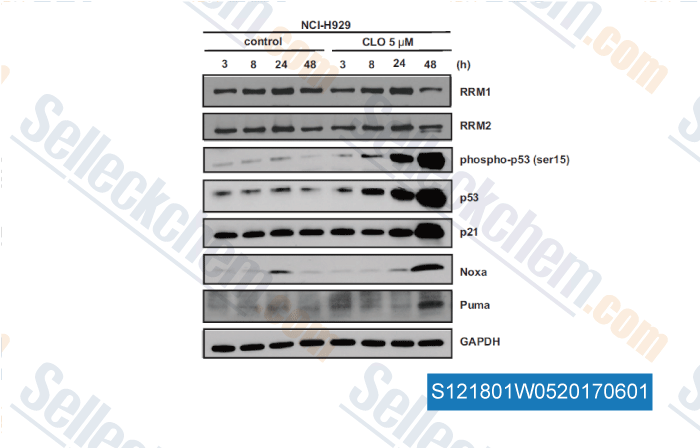
-
, , Clin Cancer Res, 2017, 23(17):5225-5237
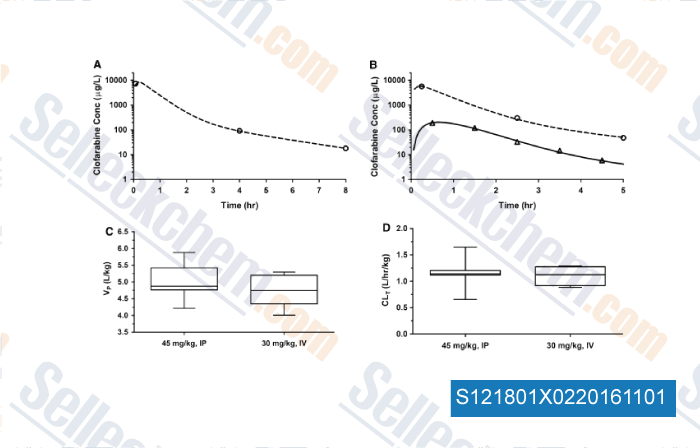
-
, , Cancer Chemoth Pharm, 2015, 75:897-906.
Selleck's Clofarabine has been cited by 20 publications
| Synergistic cytotoxicity of fludarabine, clofarabine, busulfan, vorinostat and olaparib in AML cells [ Front Oncol, 2023, 13:1287444] | PubMed: 38074694 |
| Targeting Ribonucleotide Reductase Induces Synthetic Lethality in PP2A-Deficient Uterine Serous Carcinoma [ Cancer Res, 2022, 82(4):721-733] | PubMed: 34921012 |
| Tumour cells are sensitised to ferroptosis via RB1CC1-mediated transcriptional reprogramming [ Clin Transl Med, 2022, 12(2):e747] | PubMed: 35220675 |
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
| Establishment and characterization of NCC-UPS4-C1: a novel cell line of undifferentiated pleomorphic sarcoma from a patient with Li-Fraumeni syndrome [ Hum Cell, 2022, 10.1007/s13577-022-00671-y] | PubMed: 35118583 |
| Prediction and identification of synergistic compound combinations against pancreatic cancer cells [ iScience, 2021, 24(9):103080] | PubMed: 34585118 |
| Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00579-z] | PubMed: 34304386 |
| Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00639-4] | PubMed: 34731453 |
| Establishment and characterization of NCC-MFS4-C1: a novel patient-derived cell line of myxofibrosarcoma [ Hum Cell, 2021, 34(6):1911-1918] | PubMed: 34383271 |
| Establishment and characterization of patient-derived cancer models of malignant peripheral nerve sheath tumors. [ Cancer Cell Int, 2020, 19;20:58] | PubMed: 32099531 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
