|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C25H27FN4O3 |
|||
| Molecular Weight | 450.51 | CAS No. | 288383-20-0 | |
| Solubility (25°C)* | In vitro | DMSO | 90 mg/mL (199.77 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Cediranib (AZD2171, NSC-732208) is a highly potent VEGFR(KDR) inhibitor with IC50 of <1 nM, also inhibits Flt1/4 with IC50 of 5 nM/≤3 nM, similar activity against c-Kit and PDGFRβ, 36-, 110-fold and >1000-fold selective more for VEGFR than PDGFR-α, CSF-1R and Flt3 in HUVEC cells. Cediranib (AZD2171) induces autophagic vacuole accumulation. Phase 3. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
|||||||||||
| In vitro | Cediranib inhibits VEGF-stimulated proliferation with IC50 of 0.4 nM. Cediranib suppresses PDGF-AA with IC50 of 0.04 μM in MG63 cell lines. Cediranib has been shown to block Flt1-associated kinase with IC50 of 5 nM and VEGF-C and VEGF-D receptor Flt-4 with IC50 less than 3 nM. In addition, the IC50 values for inhibition of c-Kit and PDGFRβ tyrosine kinase are 2 nM and 5 nM respectively. Furthermore, no inhibition of enzyme activity is observed when 10 μM Cediranib is assayed with 100 μM ATP against AMPK, Chk1 Akt/PKB and others. Micromolar concentrations of Cediranib are needed to prevent tumor cell proliferation in vitro. [1] | |||||||||||
| In vivo | Cediranib even suppresses tubule sprouting at subnanomolar concentrations and inhibits VEGF-induced angiogenesis. Cediranib causes hypertrophy in bone growth plate and prevents luteal development in ovary. These are physiological processes that are dependent upon angiogenesis. Cediranib shows broad spectrum activity in human tumor models at doses that are well tolerated. [1] Besides, Cediranib causes regression of vascular tissues in human lung tumor xenografts. [2] |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[1] |
|
| Animal Study:[1] |
|
References
|
Customer Product Validation
-
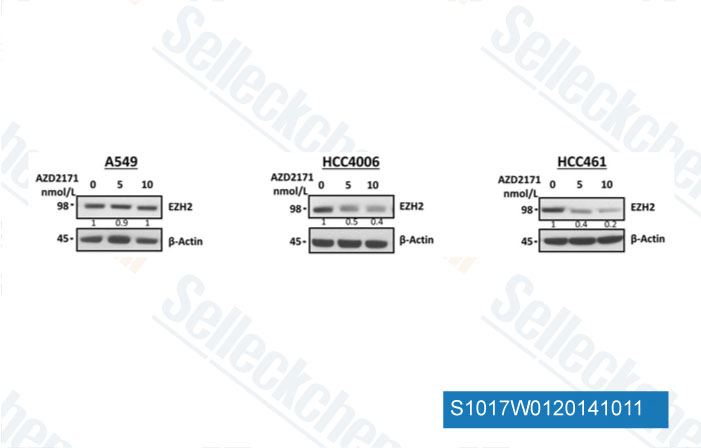
Data from [Data independently produced by Clin Cancer Res, 2014, 20, 3849-61]
-
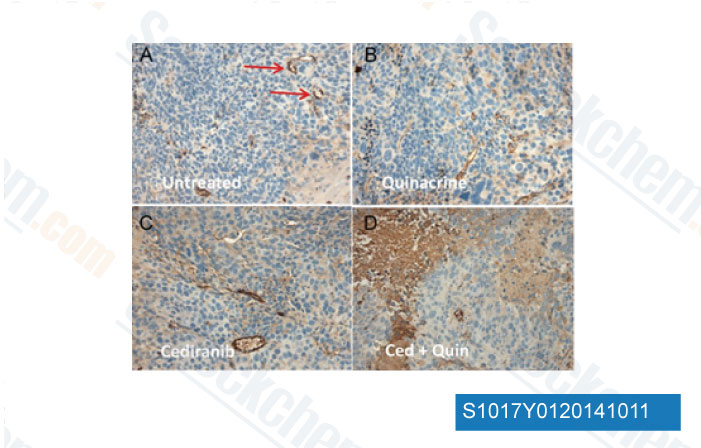
Data from [Data independently produced by Neuro Oncol, 2013, 15, 1673-83]
-
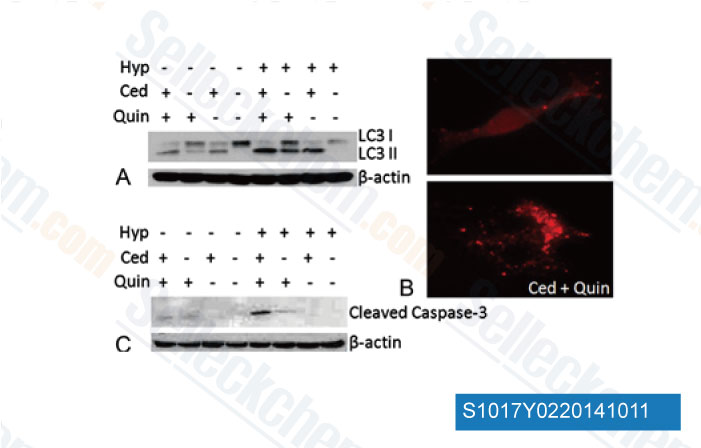
Data from [Data independently produced by Neuro Oncol, 2013, 15, 1673-83]
-
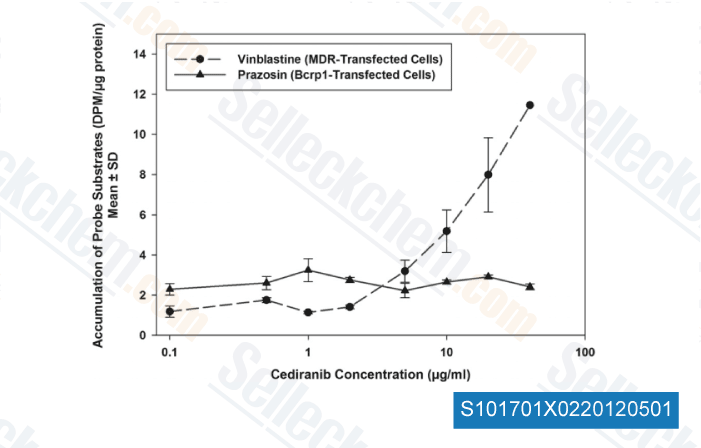
Data from [J Pharmacol Exp Ther, 2012, 341, 386-395 ]
Selleck's Cediranib (AZD2171) has been cited by 74 publications
| Bevacizumab increases the sensitivity of olaparib to homologous recombination-proficient ovarian cancer by suppressing CRY1 via PI3K/AKT pathway [ Front Oncol, 2024, 14:1302850] | PubMed: 38420012 |
| WNT5A-ROR2 axis mediates VEGF dependence of BRAF mutant melanoma [ Cell Oncol (Dordr), 2023, 46(2):391-407] | PubMed: 36539575 |
| WNT5A-ROR2 axis mediates VEGF dependence of BRAF mutant melanoma [ Cell Oncol (Dordr), 2023, 46(2):391-407] | PubMed: 36539575 |
| Relevance of the organic anion transporting polypeptide 1B3 (OATP1B3) in the personalized pharmacological treatment of hepatocellular carcinoma [ Biochem Pharmacol, 2023, 214:115681] | PubMed: 37429423 |
| Vitamin D Modulates the Response of Patient-Derived Metastatic Melanoma Cells to Anticancer Drugs [ Int J Mol Sci, 2023, 24(9)8037] | PubMed: 37175742 |
| Transforming Growth Factor Beta and Epithelial to Mesenchymal Transition Alter Homologous Recombination Repair Gene Expression and Sensitize BRCA Wild-Type Ovarian Cancer Cells to Olaparib [ Cancers (Basel), 2023, 10.3390/cancers15153919] | PubMed: 37568736 |
| Immune suppressive signaling regulated by latent transforming growth factor beta binding protein 1 promotes metastasis in cervical cancer [ Braz J Med Biol Res, 2023, 55:e12206] | PubMed: 36629522 |
| Development of an in-vitro high-throughput screening system to identify modulators of genitalia development [ PNAS Nexus, 2023, 2(1):pgac300] | PubMed: 36712925 |
| A community challenge for a pancancer drug mechanism of action inference from perturbational profile data [ Cell Rep Med, 2022, 3(1):100492] | PubMed: 35106508 |
| The multi-kinase inhibitor afatinib serves as a novel candidate for the treatment of human uveal melanoma [ Cell Oncol (Dordr), 2022, 45(4):601-619] | PubMed: 35781872 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
