|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C20H16N2O4 |
||||||
| Molecular Weight | 348.35 | CAS No. | 7689-03-4 | ||||
| Solubility (25°C)* | In vitro | DMSO | 6 mg/mL (17.22 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Camptothecin (CPT) is a specific inhibitor of DNA topoisomerase I (Topo I) with IC50 of 0.68 μM in a cell-free assay. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Phase 2. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Camptothecin (CPT), a plant alkaloid originally isolated from *Camptotheca acuminate* in 1966,[1] is noted to halt cells during the S phase of mitosis. This compound displays nanomolar potency in cytotoxicity against many human tumor cell lines, including HT29, LOX, SKOV3, and SKVLB, with IC50 values ranging from 37 nM to 48 nM.[2] In combination with TNF, it induces apoptosis in primary mouse hepatocytes, with an IC50 value of 13 μM. It also abrogated the TNF-induced NF-κB activation, as well as the expression of TNF-receptor associated factor 2 (TRAF2), X-linked inhibitor of apoptosis protein (X-IAP), and FLICE-inhibitory protein (FLIP).[4] In HCT116 cells, CPT (5 μM) induces proteasome-mediated degradation of mixed lineage leukemia 5 (MLL5) protein, which leads to phosphorylation of p53 at Ser392.[5] Due to its low solubility and adverse effects, various analogues have been developed, and two of them, topotecan and irinotecan, have been approved by the FDA and are used in cancer chemotherapy. | ||
| In vivo | Camptothecin (CPT) (8 mg/kg) displays complete growth inhibition and regression in mice xenografts of various tumors, including colon, lung, breast, stomach, and ovary tumors. [3] In mice, combinations of this compound (50 mg/kg) and TNF (5 and 7 μg/kg), but not it alone, induces liver damage. [4] |
Protocol (from reference)
| Kinase Assay:[2] |
|
|---|---|
| Cell Assay:[2] |
|
| Animal Study:[3] |
|
References
|
Customer Product Validation
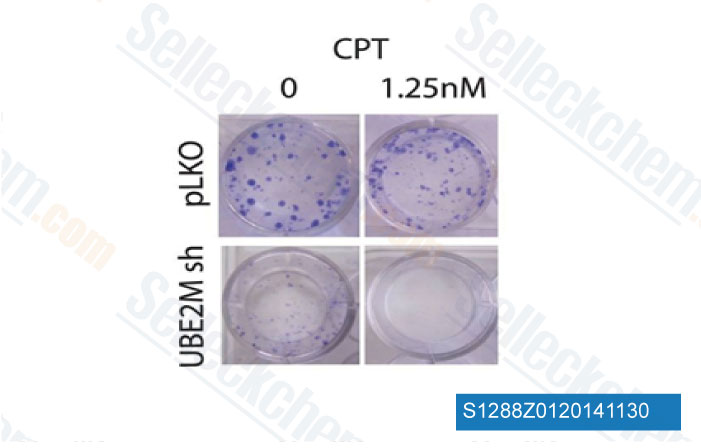
-
Data from [ PLoS One , 2014 , 9(7), e101844 ]
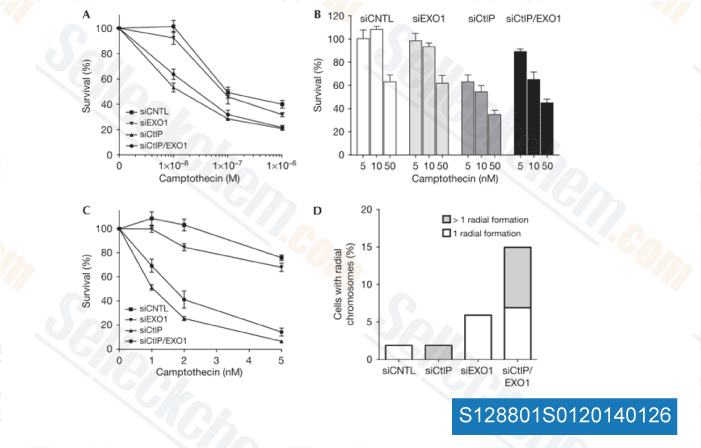
-
Data from [ EMBO Rep , 2010 , 11(12), 962-8 ]
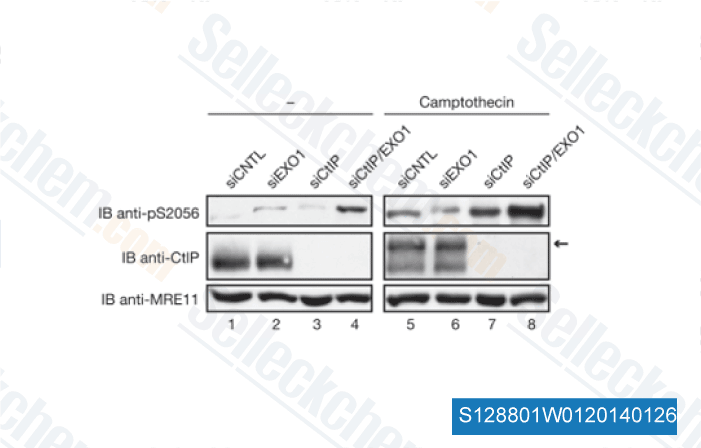
-
Data from [ EMBO Rep , 2010 , 11(12), 962-8 ]
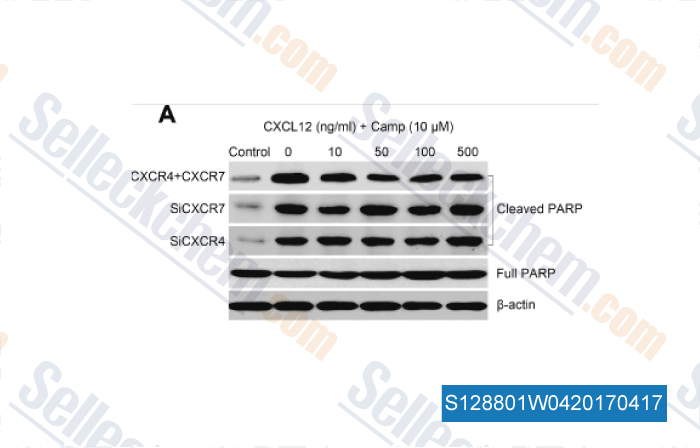
-
Data from [ , , Tumour Biol, 2016, 37(6):8169-79 ]
Selleck's Camptothecin (CPT) Has Been Cited by 169 Publications
| Non-canonical functions of DNMT3A in hematopoietic stem cells regulate telomerase activity and genome integrity [ Cell Stem Cell, 2025, S1934-5909(25)00256-5] | PubMed: 40680747 |
| Inherited deficiency of DIAPH1 identifies a DNA double strand break repair pathway regulated by γ-actin [ Nat Commun, 2025, 16(1):4491] | PubMed: 40368919 |
| A novel biosensor for the spatiotemporal analysis of STING activation during innate immune responses to dsDNA [ EMBO J, 2025, 10.1038/s44318-025-00370-y] | PubMed: 39984755 |
| DSCC1 restrains 53BP1/RIF1 signaling at DNA double-strand breaks to promote homologous recombination repair [ Cell Rep, 2025, 44(4):115452] | PubMed: 40117291 |
| Steroid-Modulated Transcription Synergistically Forms DNA Double-Strand Breaks With Topoisomerase II Inhibitor [ Cancer Sci, 2025, 10.1111/cas.70081] | PubMed: 40231641 |
| The novel role of LCK and other PcDEGs in the diagnosis and prognosis of sepsis: Insights from bioinformatic identification and experimental validation [ Int Immunopharmacol, 2025, 149:114194] | PubMed: 39904039 |
| The Kinase Inhibitor GNF-7 Is Synthetically Lethal in Topoisomerase 1-Deficient Ewing Sarcoma [ Cancers (Basel), 2025, 17(15)2475] | PubMed: 40805174 |
| Peroxiredoxin 1 inhibits tumorigenesis by activating the NLRP3/GSDMD pathway to induce pyroptosis of colorectal cancer cells [ World J Gastroenterol, 2025, 31(36):111557] | PubMed: 41025079 |
| Discovery and Characterization of Small Molecule Inhibitors Targeting Exonuclease 1 for Homologous Recombination-Deficient Cancer Therapy [ ACS Chem Biol, 2025, 10.1021/acschembio.5c00117] | PubMed: 40378357 |
| DDX18 influences chemotherapy sensitivity in colorectal cancer by regulating genomic stability [ Exp Cell Res, 2025, 444(1):114344] | PubMed: 39577603 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
