|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C20H16N2O4 |
|||
| Molecular Weight | 348.35 | CAS No. | 7689-03-4 | |
| Solubility (25°C)* | In vitro | 0.1M NaOH | 2.5 mg/mL (7.17 mM) | |
| DMSO | Insoluble | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Camptothecin (CPT) is a specific inhibitor of DNA topoisomerase I (Topo I) with IC50 of 0.68 μM in a cell-free assay. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Phase 2. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Camptothecin, a plant alkaloid orignially isolated from Camptotheca acuminate in 1966. [1] Camptothecin is noted to halt cells during the S phase of mitosis. Camptothecin displays nanomolar potency in cytotoxicity against many human tumor cell lines, including HT29, LOX, SKOV3, and SKVLB, with IC50 values ranging from 37 nM to 48 nM. [2] In combination with TNF, Camptothecin induces apoptosis in primary mouse hepatocytes, with an IC50 value of 13 μM. Camptothecin also abrogated the TNF-induced NF-κB Activation, as well as the expression of TNF-receptor associated factor 2 (TRAF2), X-linked inhibitor of apoptosis protein (X-IAP), and FLICE-inhibitory protein (FLIP). [4] In HCT116 cells, Camptothecin (5 μM) induces proteasome-mediated degradation of mixed lineage leukemia 5 (MLL5) protein, which leads to phosphorylation of p53 at Ser392. [5] Due to the low solubility and adverse effects of Camptothecin, various Camptothecin analogues have been developed, and two of them, topotecan and irinotecan, has been approved by FDA and are used in cancer chemotherapy. | ||
| In vivo | Camptothecin (8 mg/kg) displays complete growth inhibition and regression in mice xenografts of various tumors, including colon, lung, breast, stomach, and ovary tumors. [3] In mice, combinations of Camptothecin (50 mg/kg) and TNF (5 and 7 μg/kg), but not Camptothecin alone, induces liver damage. [4] |
Protocol (from reference)
| Kinase Assay:[2] |
|
|---|---|
| Cell Assay:[2] |
|
| Animal Study:[3] |
|
References
Customer Product Validation
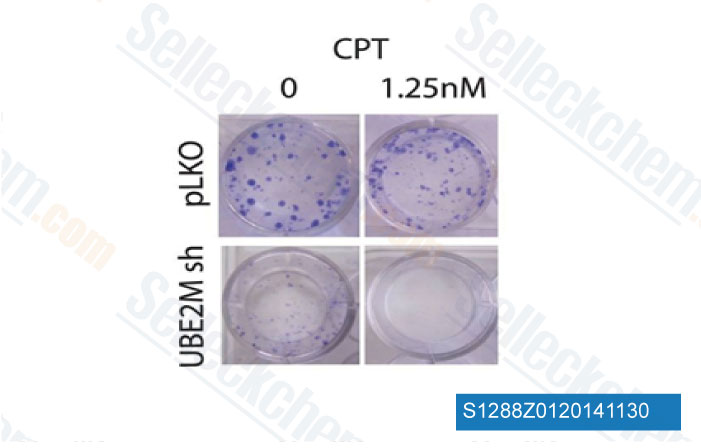
-
Data from [Data independently produced by PLoS One, 2014, 9(7), e101844]
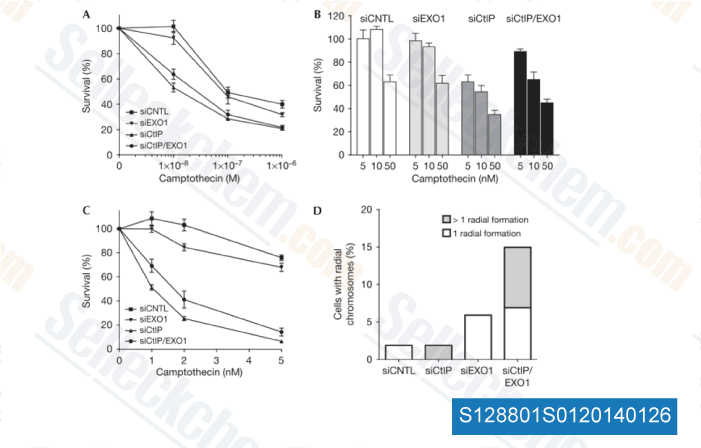
-
Data from [EMBO Rep, 2010, 11(12), 962-8]
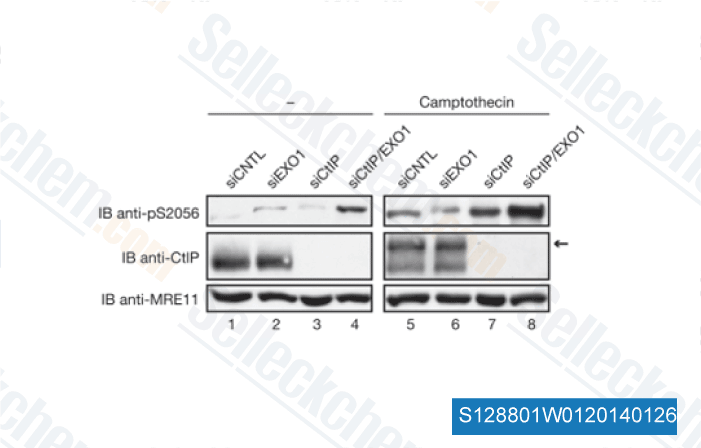
-
Data from [EMBO Rep, 2010, 11(12), 962-8]
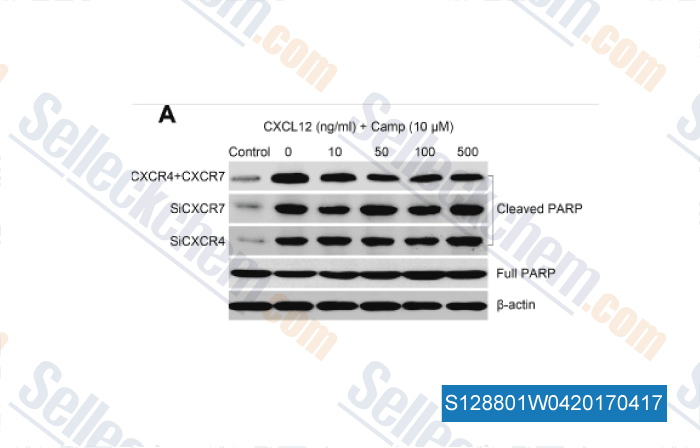
-
Data from [Data independently produced by , , Tumour Biol, 2016, 37(6):8169-79]
Selleck's Camptothecin (CPT) has been cited by 156 publications
| The novel role of LCK and other PcDEGs in the diagnosis and prognosis of sepsis: Insights from bioinformatic identification and experimental validation [ Int Immunopharmacol, 2025, 149:114194] | PubMed: 39904039 |
| DDX18 influences chemotherapy sensitivity in colorectal cancer by regulating genomic stability [ Exp Cell Res, 2025, 444(1):114344] | PubMed: 39577603 |
| H2AX promotes replication fork degradation and chemosensitivity in BRCA-deficient tumours [ Nat Commun, 2024, 15(1):4430] | PubMed: 38789420 |
| UBA1 inhibition sensitizes cancer cells to PARP inhibitors [ Cell Rep Med, 2024, 5(12):101834] | PubMed: 39626673 |
| SIRT1 ISGylation accelerates tumor progression by unleashing SIRT1 from the inactive state to promote its deacetylase activity [ Exp Mol Med, 2024, 56(3):656-673] | PubMed: 38443596 |
| Human PC4 supports telomere stability and viability in cells utilizing the alternative lengthening of telomeres mechanism [ EMBO Rep, 2024, ] | PubMed: 39468351 |
| Dual targeting of the androgen receptor and PI3K/AKT/mTOR pathways in prostate cancer models improves antitumor efficacy and promotes cell apoptosis [ Mol Oncol, 2024, 10.1002/1878-0261.13577] | PubMed: 38225213 |
| Metachromin C, a marine-derived natural compound, shows potential in antitumor activity [ Int J Med Sci, 2024, 21(13):2578-2594] | PubMed: 39439453 |
| EZH2 directly methylates PARP1 and regulates its activity in cancer [ Sci Adv, 2024, 10(48):eadl2804] | PubMed: 39602541 |
| Epigenetic targeting of PGBD5-dependent DNA damage in SMARCB1-deficient sarcomas [ bioRxiv, 2024, 2024.05.03.592420] | PubMed: 38766189 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
