|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C19H25BN4O4 |
|||
| Molecular Weight | 384.24 | CAS No. | 179324-69-7 | |
| Solubility (25°C)* | In vitro | DMSO | 76 mg/mL (197.79 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Bortezomib is a potent 20S proteasome inhibitor with Ki of 0.6 nM. It exhibits favorable selectivity towards tumor cells over normal cells. Bortezomib (PS-341) inhibits NF-κB and induces ERK phosphorylation to suppress cathepsin B and inhibit the catalytic process of autophagy in ovarian cancer and other solid tumors. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Bortezomib, a boronic acid dipeptide, is a highly selective, reversible inhibitor of the 26S proteasome which primarily functions in the degradation of mis-folded proteins and is essential for the regulation of the cell cycle. Exposure to Bortezomib has been shown to stabilize p21, p27, and p53, as well as the proapoptotic Bid and Bax proteins, caveolin-1, and inhibitor κB-α, which prevents activation of nuclear factor κB-induced cell survival pathways. Bortezomib also promotes the activation of the proapoptotic c-Jun-NH2 terminal kinase, as well as the endoplasmic reticulum stress response. Alteration of the levels of these cellular proteins leads to inhibition of proliferation, migration, and promotion of apoptosis of cancer cells. [2] Bortezomib is shown to penetrate into cells and inhibit proteasome-mediated intracellular proteolysis of long-lived proteins with a concentration that inhibits 50% of the proteolysis of ∼0.1 μM. The average growth inhibition of 50% value for Bortezomib across the entire panel of 60 cancer cell lines derived from multiple human tumors from the US National Cancer Institute (NCI) is 7 nM. Treatment of PC-3 cells with Bortezomib (100 nM) for 8 h results in the accumulation of cells in G2-M, with a corresponding decrease in the number of cells in G1. Bortezomib kills PC-3 cells at 24 and 48 hr with IC50 of 100 and 20 nM, respectively. Bortezomib induces nuclear condensation at 16–24 hr after treatment. Bortezomib treatment leads to PARP cleavage in a time-dependent manner with concentrations as low as 100 nM being effective at 24 hr. [1] |
||||
| In vivo | The anticancer effects of bortezomib as a single agent have been demonstrated in xenograft models of multiple myeloma, adult T-cell leukemia, lung, breast, prostate, pancreatic, head and neck, and colon cancer, and in melanoma. [2] Oral bortezomib 1.0 mg/ kg daily for 18 days causes tumor growth delays, as well as a decrease in the number of metastases in the Lewis lung cancer model. Bortezomib at a single dose of up to 5 mg/kg significantly decreased the surviving fraction of breast tumor cells. Bortezomib 1.0 mg/kg administrated weekly for 4 weeks reduces tumor growth by 60% in murine xenograft models of prostate cancer. 1.0 mg/kg Bortezomib administration for 4 weeks results in a 72% or 84% reduction in pancreatic cancer murine xenografts growth, as well as an increase in tumor cell apoptosis. 1.0 mg/kg Bortezomib treatment results in significant inhibition of human plasmacytoma xenograft growth, increase in tumor cells apoptosis and overall survival, and a decrease in tumor angiogenesis. [3] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
Customer Product Validation
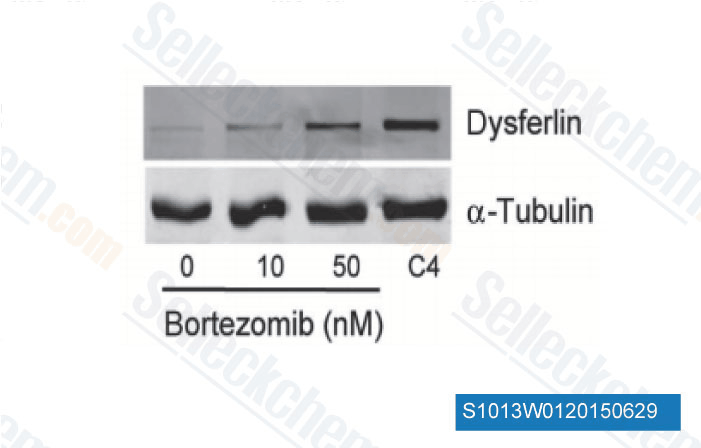
-
Data from [Data independently produced by Sci Transl Med, 2015, 6(250), 250ra112]
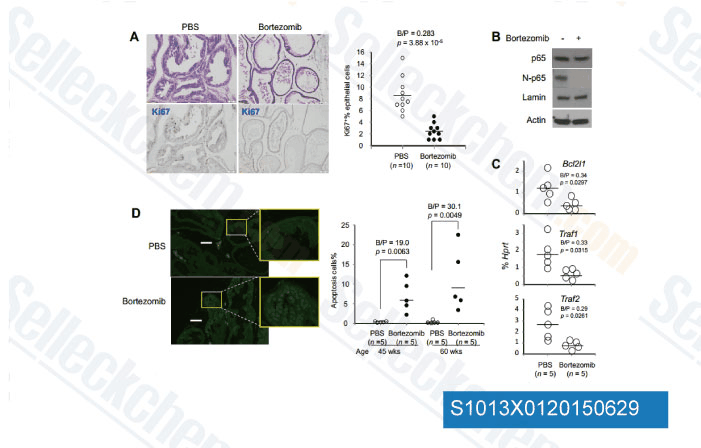
-
Data from [Data independently produced by Cancer Res, 2015, 75(8), 1714-24]
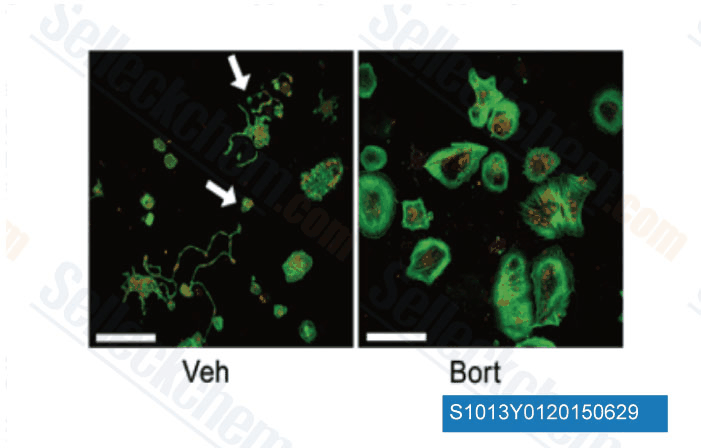
-
Data from [Data independently produced by J Clin Invest, 2014, 124(9), 3757-66]
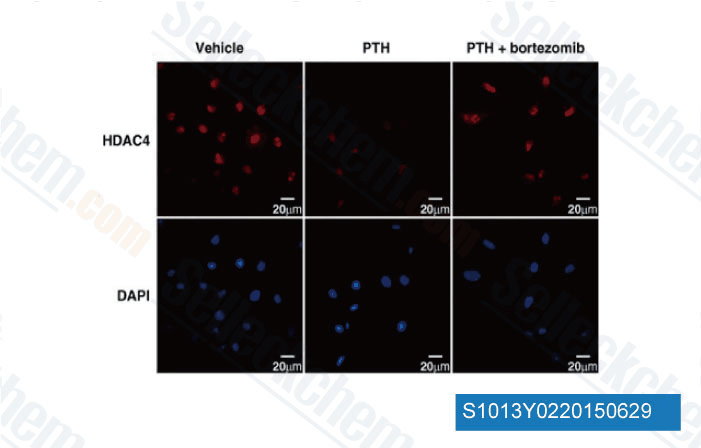
-
Data from [Data independently produced by J Cell Biol, 2014, 205(6), 771-80]
Selleck's Bortezomib has been cited by 1158 publications
| Neoadjuvant PARPi or chemotherapy in ovarian cancer informs targeting effector Treg cells for homologous-recombination-deficient tumors [ Cell, 2024, 187(18):4905-4925.e24] | PubMed: 38971151 |
| Global, site-resolved analysis of ubiquitylation occupancy and turnover rate reveals systems properties [ Cell, 2024, S0092-8674(24)00315-5] | PubMed: 38626770 |
| Neoadjuvant PARPi or chemotherapy in ovarian cancer informs targeting effector Treg cells for homologous-recombination-deficient tumors [ Cell, 2024, S0092-8674(24)00653-6] | PubMed: 38971151 |
| Global, site-resolved analysis of ubiquitylation occupancy and turnover rate reveals systems properties [ Cell, 2024, 187(11):2875-2892.e21] | PubMed: 38626770 |
| Tet methylcytosine dioxygenase 2 (TET2) deficiency elicits EGFR-TKI (tyrosine kinase inhibitors) resistance in non-small cell lung cancer [ Signal Transduct Target Ther, 2024, 9(1):65] | PubMed: 38461173 |
| Proteasome inhibition enhances the anti-leukemic efficacy of chimeric antigen receptor (CAR) expressing NK cells against acute myeloid leukemia [ J Hematol Oncol, 2024, 17(1):85] | PubMed: 39285441 |
| Nuclear proteasomes buffer cytoplasmic proteins during autophagy compromise [ Nat Cell Biol, 2024, 10.1038/s41556-024-01488-7] | PubMed: 39209961 |
| Recruitment of Cdc48 to chloroplasts by a UBX-domain protein in chloroplast-associated protein degradation [ Nat Plants, 2024, 10(9):1400-1417] | PubMed: 39160348 |
| Combined KRAS-MAPK pathway inhibitors and HER2-directed drug conjugate is efficacious in pancreatic cancer [ Nat Commun, 2024, 15(1):2503] | PubMed: 38509064 |
| Engineered Cas9 variants bypass Keap1-mediated degradation in human cells and enhance epigenome editing efficiency [ Nucleic Acids Res, 2024, gkae761] | PubMed: 39228373 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
