|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C40H50N8O6 |
|||
| Molecular Weight | 738.88 | CAS No. | 1009119-64-5 | |
| Solubility (25°C)* | In vitro | DMSO | 148 mg/mL (200.3 mM) | |
| Ethanol | 148 mg/mL (200.3 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Daclatasvir (BMS-790052, EBP883) is a highly selective inhibitor of HCV NS5A with EC50 of 9-50 pM, for a broad range of HCV replicon genotypes and the JFH-1 genotype 2a infectious virus in cell culture. Phase 3. | ||
|---|---|---|---|
| Targets |
|
||
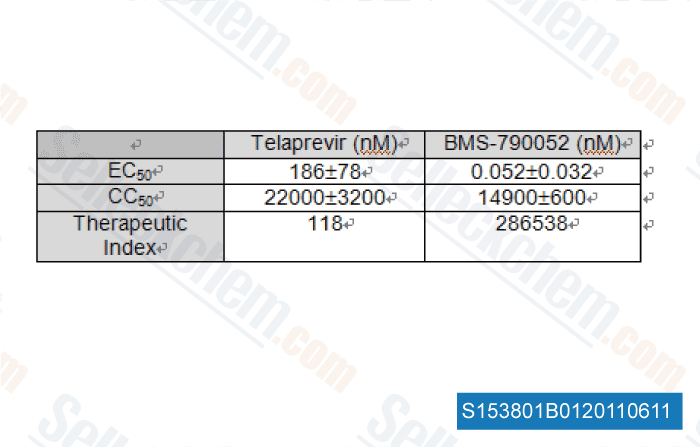
| In vitro | BMS-790052 is one of the most potent inhibitors of HCV replication reported so far. The mean EC50 valuses of BMS-790052 are 50 and 9 pM for HCV genotype 1a and 1b replicons, respectively. BMS-790052 displays a therapeutic index (CC50/EC50) of at least 105 and is inactive towards a panel of 10 RNA and DNA viruses, with EC50 higher than 10 μM. This confirms BMS-790052's specificity for HCV. [1] In Huh7 cells harboring the HCV genotype 1b replicons, BMS-790052 blocks both transient and stable HCV genome replication, with EC50 values raging from 1-15 pM. BMS-790052 (100 pM or 1 nM) has been shown to alter the subcellular localization and biochemical fractionation of NS5A. [2] BMS-790052 inhibits hybrid replicons containing HCV genotype-4 NS5A genes with EC50 of 7-13 pM. Residue 30 of NS5A is an important site for BMS-790052-mediated resistance in the hybrid replicons. [3] | ||
| Features | First-in-class, highly selective inhibitor of hepatitis C virus (HCV) NS5A with picomolar EC50 values. |
Protocol (from reference)
| Kinase Assay:[4] |
|
|---|---|
| Cell Assay:[1] |
|
References
Customer Product Validation
-
, 2011, Dr. Julie Sheldon,Dr Esteban Domingo and Dr Celia Perales
-
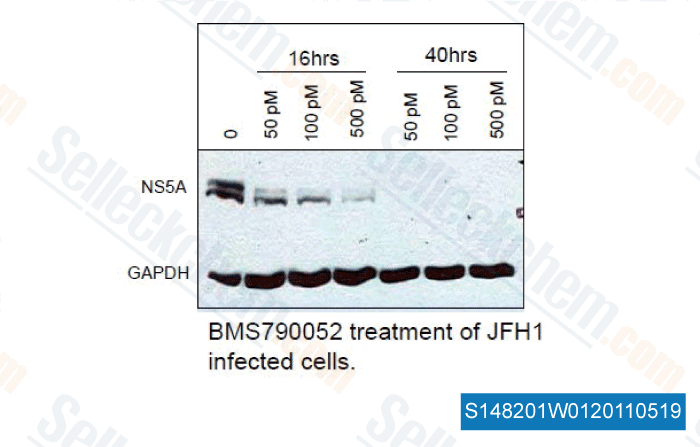
, 2011, Dr. Anita Nag of Florida State University
-
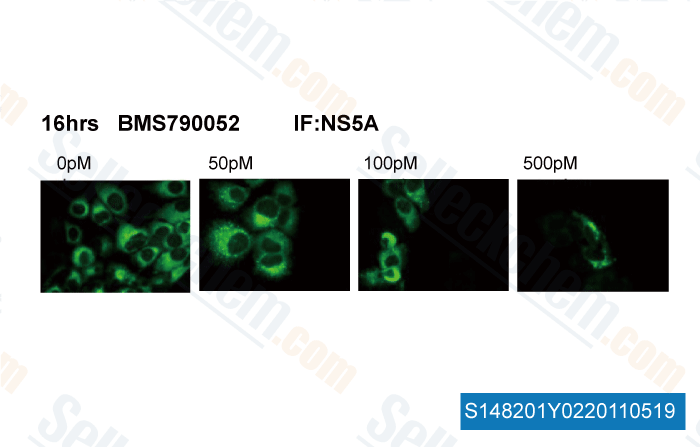
, 2011, Dr. Anita Nag of Florida State University
-
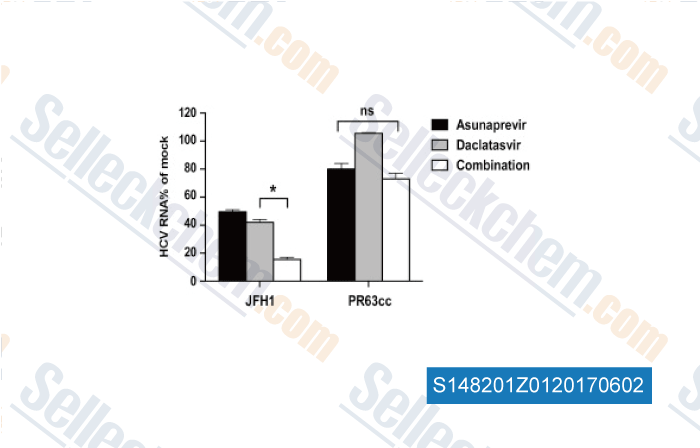
Data from [Data independently produced by , , Antiviral Res, 2017, 139:18-24]
Selleck's Daclatasvir (BMS-790052) has been cited by 56 publications
| Hepatitis C virus-induced differential transcriptional traits in host cells after persistent infection elimination by direct-acting antivirals in cell culture [ J Med Virol, 2024, 96(7):e29787] | PubMed: 38988177 |
| Antiviral drugs prolong survival in murine recessive dystrophic epidermolysis bullosa [ EMBO Mol Med, 2024, 16(4):870-884] | PubMed: 38462666 |
| Unraveling the dynamics of hepatitis C virus adaptive mutations and their impact on antiviral responses in primary human hepatocytes [ J Virol, 2024, e0192123.] | PubMed: 38319104 |
| Mechanisms of Action of the Host-Targeting Agent Cyclosporin A and Direct-Acting Antiviral Agents against Hepatitis C Virus [ Viruses, 2023, 15(4)981] | PubMed: 37112961 |
| Hepatitis C virus cell culture adaptive mutations enhance cell culture propagation by multiple mechanisms but boost antiviral responses in primary human hepatocytes [ bioRxiv, 2023, 2023.11.22.568224] | PubMed: 38045248 |
| Combination of antiviral drugs inhibits SARS-CoV-2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture [ Commun Biol, 2022, 5(1):154] | PubMed: 35194144 |
| Going Viral: An Investigation into the Chameleonic Behaviour of Antiviral Compounds [ Chemistry, 2022, e202202798.] | PubMed: 36286339 |
| Correlation of hepatitis C virus-mediated endoplasmic reticulum stress with autophagic flux impairment and hepatocarcinogenesis [ Med Mol Morphol, 2021, 10.1007/s00795-020-00271-5] | PubMed: 33386512 |
| Combination of Antiviral Drugs to Inhibit SARS-CoV-2 Polymerase and Exonuclease as Potential COVID-19 Therapeutics [ bioRxiv, 2021, 2021.07.21.453274] | PubMed: 34312622 |
| Fluoxazolevir inhibits hepatitis C virus infection in humanized chimeric mice by blocking viral membrane fusion [ Nat Microbiol, 2020, 10.1038/s41564-020-0781-2] | PubMed: 32868923 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
