|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C8H12N4O5 |
||||||
| Molecular Weight | 244.2 | CAS No. | 320-67-2 | ||||
| Solubility (25°C)* | In vitro | DMSO | 49 mg/mL (200.65 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Azacitidine (5-Azacytidine, 5-AzaC, Ladakamycin, AZA, 5-Aza, CC-486,NSC 102816) is a nucleoside analogue of cytidine that specifically inhibits DNA methylation by trapping DNA methyltransferases. Azacitidine induces mitochondrial apoptosis and autophagy. | |
|---|---|---|
| Targets |
|
|
| In vitro | Azacitidine is widely used to demonstrate the correlation between loss of methylation in specifc gene regions and activation of the associated genes. After incorporation into DNA, Azacitidine inhibits DNA methyltransferase noncompetitively, causing a block in cytosine methylation in newly replicated DNA but not in resting, nondividing cells. [1] Azacitidine induces differentiation of Friend Erythroleukemia Cell C3H10T1/2 with myotube formation. [2] Azacitidine can be activated to the nucleoside triphosphate and incorporate into both DNA and RNA, leading to inhibition of DNA, RNA and protein synthesis in normal eukaryotic cells and in cancer cell lines, which could finally leads to cell death. Azacitidine also inhibits the incorporation of purine metabolites into macromolecules. Azacitidine inhibits the L1210 cells growth with IC50 and of 0.019 μg/mL. [3] |
|
| In vivo | Azacitidine inhibits polynucleotide synthesis in leukemic BDF1 mice. [3] Azacitidine (3 mg/kg, i.p.) increases the mean survival time in leukemic BDF1 mice inoculated with Ll210 ascites tumor cells. Azacitidine markedly suppresses all enzymes activity in the polyamine-biosynthetic pathway, including ornithine decarboxylase activity. putrescine-dependent S-adenosyl-L-methionine decarboxylase activity, and spermidine-dependent S-adenosyl-L-methionine decarboxylase activity. Azacitidine also inhibits the accumulations of polyamines in leukemic mice. [4] |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
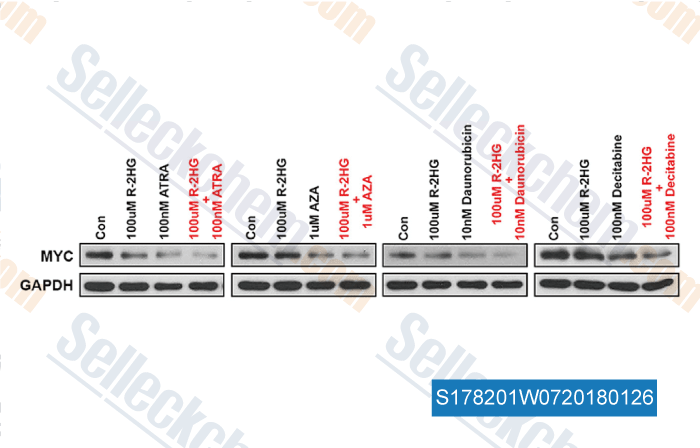
-
, , Cell, 2018, 172(1-2):90-105
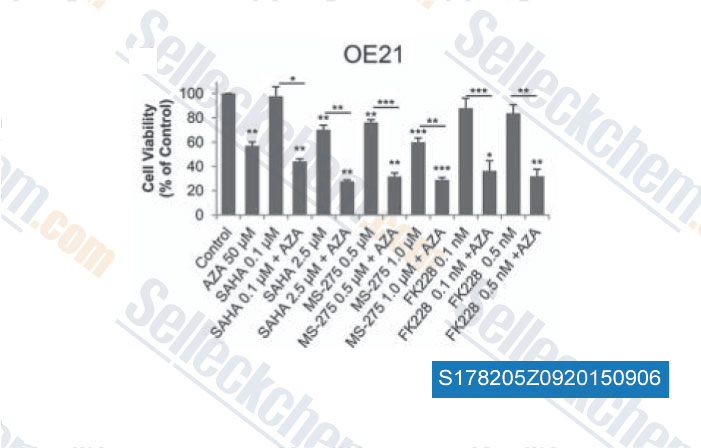
-
Data from [Data independently produced by , , Epigenetics, 2015, 10(5): 431-45]
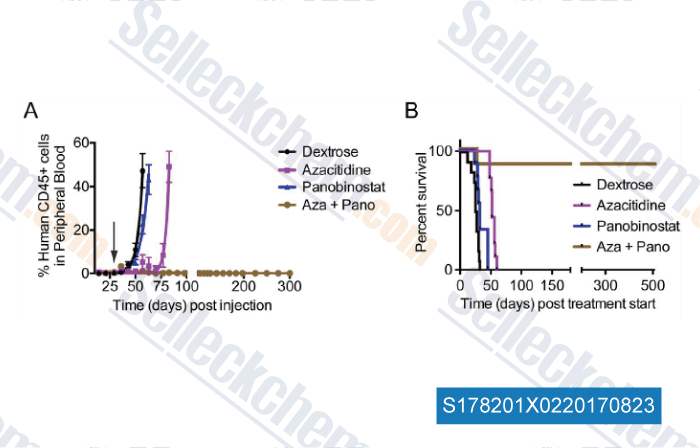
-
Data from [Data independently produced by , , Leuk Res, 2017, 58:91-97]
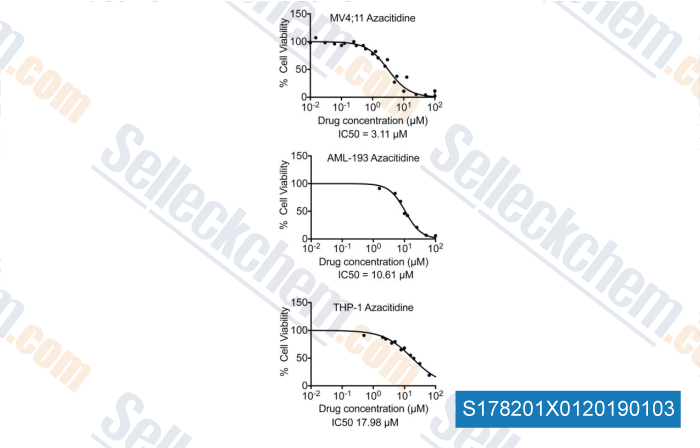
-
Data from [Data independently produced by , , Leuk Res, 2018, 58:91-97]
Selleck's Azacitidine (5-Azacytidine) has been cited by 143 publications
| A patient-derived T cell lymphoma biorepository uncovers pathogenetic mechanisms and host-related therapeutic vulnerabilities [ Cell Rep Med, 2025, S2666-3791(25)00102-8] | PubMed: 40147445 |
| ONC213: a novel strategy to resensitize resistant AML cells to venetoclax through induction of mitochondrial stress [ J Exp Clin Cancer Res, 2025, 44(1):10] | PubMed: 39780285 |
| The integrative genomic and functional immunological analyses of colorectal cancer initiating cells to modulate stemness properties and the susceptibility to immune responses [ J Transl Med, 2025, 23(1):193] | PubMed: 39962504 |
| TFPI2 hypermethylation promotes diabetic atherosclerosis progression through the Ap2α/PPARγ axis [ J Mol Cell Cardiol, 2025, 198:45-59] | PubMed: 39631358 |
| A high-throughput screening platform to identify MYCN expression inhibitors for liver cancer therapy [ Front Oncol, 2025, 15:1486671] | PubMed: 40027135 |
| Reciprocal regulation between DNMT3A/3B and microRNAs miRs-299-3p/-30e is a causal factor for the downregulation of microRNAs targeting androgen receptor in prostate cancer [ Heliyon, 2025, 11(3):e41948] | PubMed: 39944334 |
| Proteasome inhibition enhances the anti-leukemic efficacy of chimeric antigen receptor (CAR) expressing NK cells against acute myeloid leukemia [ J Hematol Oncol, 2024, 17(1):85] | PubMed: 39285441 |
| TOPORS E3 ligase mediates resistance to hypomethylating agent cytotoxicity in acute myeloid leukemia cells [ Nat Commun, 2024, 15(1):7360] | PubMed: 39198401 |
| Orthogonal proteogenomic analysis identifies the druggable PA2G4-MYC axis in 3q26 AML [ Nat Commun, 2024, 15(1):4739] | PubMed: 38834613 |
| S-nitrosothiol homeostasis maintained by ADH5 facilitates STING-dependent host defense against pathogens [ Nat Commun, 2024, 15(1):1750] | PubMed: 38409248 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
