|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C26H33NO2 |
|||
| Molecular Weight | 391.55 | CAS No. | 154229-18-2 | |
| Solubility (25°C)* | In vitro | Ethanol | 78 mg/mL (199.2 mM) | |
| DMSO | Insoluble | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Abiraterone Acetate is an acetate salt form of Abiraterone which is a steroidal cytochrome CYP17 inhibitor with IC50 of 72 nM in a cell-free assay. Abiraterone acetate is an oral androgen biosynthesis inhibitor. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Abiraterone shows a good complexation with the heme iron only in SM1. [1] Abiraterone blocks the synthesis of androgens by inhibiting CYP17A1. Abiraterone also blocks 3β-hydroxysteroid dehydrogenase (3βHSD), an enzyme that is absolutely required for the synthesis of biologically active androgens. Abiraterone inhibits conversion of DHEA to Δ4-androstenedione. Abiraterone inhibition of 3βHSD blocks DHT synthesis and the androgen receptor response. Abiraterone inhibits the conversion of Δ5-androstenediol to testosterone. [2] Abiraterone inhibits C17,20-lyase, with an IC50 of 5.8 nM, in rat testis microsomes. Abiraterone significantly inhibits testosterone secretion (−48%) and in turn increases LH concentration (192%). [3] Abiraterone inhibits in vitro proliferation and AR-regulated gene expression of AR-positive prostate cancer cells, which could be explained by AR antagonism in addition to inhibition of steroidogenesis. [4] |
||
| In vivo | Following intraperitoneal administration in a rodent model, abiraterone was found to have rapid deacetylation. When administered as its acetate pro-drug (CB 7630), it suppressed circulating testosterone to undetectable levels and markedly decreased the weights of androgen sensitive organs. Abiraterone is well tolerated and the mean elimination half-life of abiraterone in these studies was 27.6 h (thus supporting the use of once-daily dosing)[5]. Preclinical studies with abiraterone demonstrated reduction in androgen production downstream of CYP17 which resulted in decreased weight of the ventral prostate, testis, and seminal vesicles in mice[6]. |
||
| Features | Abiraterone is a drug used in castration-resistant prostate cancer. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
Customer Product Validation
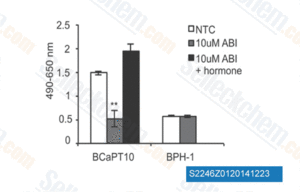
-
Data from [Data independently produced by Endocrinology, 2014, 155(2), 358-69]
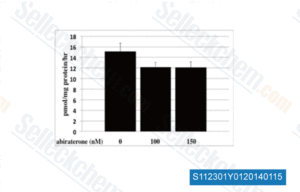
-
Data from [Data independently produced by Prostate, 2013, 74, 235-49]
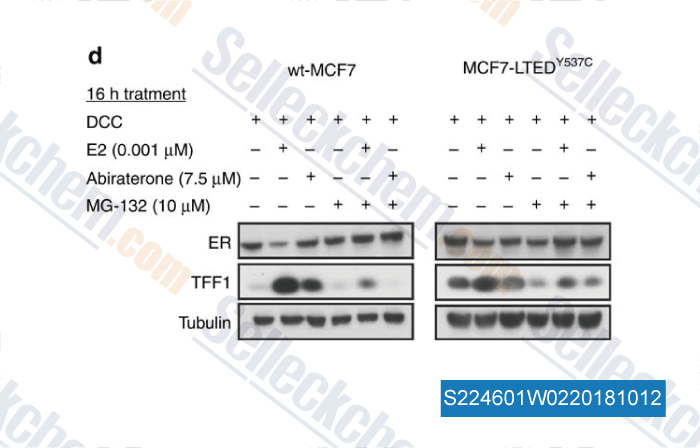
-
Data from [Data independently produced by , , Br J Cancer, 2018, doi:10.1038/s41416-018-0158-y]
Selleck's Abiraterone Acetate has been cited by 35 publications
| The Role of CENPK Splice Variant in Abiraterone Response in Metastatic Castration-Resistant Prostate Cancer [ Cells, 2024, 13(19)1622] | PubMed: 39404386 |
| Plk1 Inhibitors and Abiraterone Synergistically Disrupt Mitosis and Kill Cancer Cells of Disparate Origin Independently of Androgen Receptor Signaling [ Cancer Res, 2023, 83(2):219-238] | PubMed: 36413141 |
| Androgen receptor blockade resistance with enzalutamide in prostate cancer results in immunosuppressive alterations in the tumor immune microenvironment [ J Immunother Cancer, 2023, 11(5)e006581] | PubMed: 37147019 |
| Allosteric inhibition of HSP70 in collaboration with STUB1 augments enzalutamide efficacy in antiandrogen resistant prostate tumor and patient-derived models [ Pharmacol Res, 2023, 189:106692] | PubMed: 36773708 |
| Novel inhibition of AKR1C3 and androgen receptor axis by PTUPB synergizes enzalutamide treatment in advanced prostate cancer [ Oncogene, 2023, 42(9):693-707] | PubMed: 36596844 |
| Novel inhibition of AKR1C3 and androgen receptor axis by PTUPB synergizes enzalutamide treatment in advanced prostate cancer [ Oncogene, 2023, 42(9):693-707] | PubMed: 36596844 |
| Loss of Long Noncoding RNA NXTAR in Prostate Cancer Augments Androgen Receptor Expression and Enzalutamide Resistance [ Cancer Res, 2022, 82(1):155-168] | PubMed: 34740892 |
| miR-143 mediates abiraterone acetate resistance by regulating the JNK/Bcl-2 signaling pathway in prostate cancer [ J Cancer, 2022, 13(15):3652-3659] | PubMed: 36606191 |
| Abiraterone acetate induces CREB1 phosphorylation and enhances the function of the CBP-p300 complex, leading to resistance in prostate cancer cells [ Clin Cancer Res, 2021, clincanres.4391.2020] | PubMed: 33495313 |
| Antiproliferative, proapoptotic, and tumor-suppressing effects of the novel anticancer agent alsevirone in prostate cancer cells and xenografts [ Arch Pharm (Weinheim), 2021, e2100316] | PubMed: 34668210 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
