research use only
MEK162 (Binimetinib, ARRY-162) MEK inhibitor
Cat.No.S7007
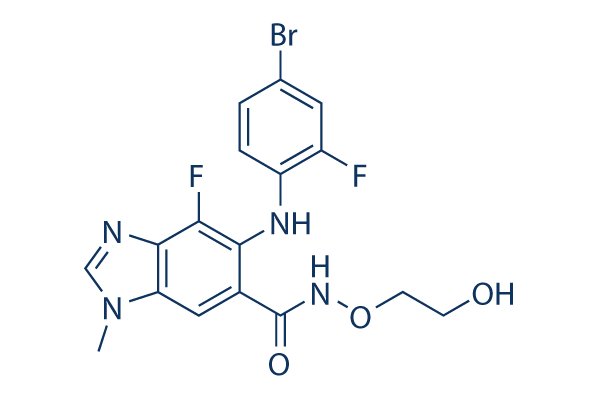
Chemical Structure
Molecular Weight: 441.23
Quality Control
| Related Targets | ERK p38 MAPK Raf JNK Ras KRas S6 Kinase MAP4K TAK1 Mixed Lineage Kinase |
|---|---|
| Other MEK Inhibitors | PD0325901 (Mirdametinib) U0126-EtOH PD 98059 PD184352 (CI-1040) BIX 02189 Pimasertib (AS-703026) Refametinib (RDEA119) TAK-733 AZD8330 BIX 02188 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| NCI-H727 | Function assay | IC50=115 nM | 30352565 | |||
| Mel IL | Cytotoxicity assay | IC50=3.7 ± 0.2 μM | 30551515 | |||
| Mel IL/R | Cytotoxicity assay | IC50=2.7 ± 0.3 μM | 30551515 | |||
| Mel Z | Cytotoxicity assay | IC50=3.8 ± 0.2 μM | 30551515 | |||
| A375 | Cytotoxicity assay | IC50=9.8 ± 0.1 μM | 30551515 | |||
| Mel Me | Cytotoxicity assay | IC50=13.3 ± 0.3 μM | 30551515 | |||
| Mel MTP | Cytotoxicity assay | IC50=10.2 ± 0.4 μM | 30551515 | |||
| U2OS cells | Function assay | 1 μM | MEK162 blocked ERK activation (p-ERK1/2) in CZ415-treated U2OS cells | 29137241 | ||
| CHP-212 | Cell viability assay | 120 h | IC50=0.0083 μM | 26925841 | ||
| SK-N-AS | Cell viability assay | 120 h | IC50=0.067 μM | 26925841 | ||
| SK-N-BE(2) | Cell viability assay | 120 h | IC50=0.28 μM | 26925841 | ||
| SJ-NB-10 | Cell viability assay | 120 h | IC50=1.16 μM | 26925841 | ||
| CHP-134 | Cell viability assay | 120 h | IC50>15 μM | 26925841 | ||
| Kelly | Cell viability assay | 120 h | IC50>15 μM | 26925841 | ||
| LAN-5 | Cell viability assay | 120 h | IC50>15 μM | 26925841 | ||
| NGP | Cell viability assay | 120 h | IC50>15 μM | 26925841 | ||
| SK-N-DZ | Cell viability assay | 120 h | IC50>15 μM | 26925841 | ||
| A549 | Cell cycle assay | 0, 0.5, 1 μM | 48h | at relatively low concentration ranges ≤ 1 μM (e.g., 0.5 and 1 μM) induced G1 arrest | 25937299 | |
| H157 | Cell cycle assay | 0, 0.5, 1 μM | 48h | at relatively low concentration ranges ≤ 1 μM (e.g., 0.5 and 1 μM) induced G1 arrest | 25937299 | |
| H522 | Cell cycle assay | 0, 0.5, 1 μM | 48h | at relatively low concentration ranges ≤ 1 μM (e.g., 0.5 and 1 μM) induced G1 arrest | 25937299 | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 88 mg/mL
(199.44 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 441.23 | Formula | C17H15BrF2N4O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 606143-89-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | ARRY-162,ARRY-438162 | Smiles | CN1C=NC2=C1C=C(C(=C2F)NC3=C(C=C(C=C3)Br)F)C(=O)NOCCO | ||
Mechanism of Action
| Targets/IC50/Ki |
MEK
(Cell-free assay) 12 nM
|
|---|---|
| In vitro |
Binimetinib (MEK162) is a recently disclosed potent and selective ATP non-competitive MEK1/2 inhibitor, inhibits pERK in cells with an IC50 of11 nM. This compound (625 nM) inhibits in vitro osteoclast differentiation with IC50 of 39 nM. It (10 μM) inhibits in vitro osteoclast resorption with IC50 of 625 nM. It (2 μM) weakly affects osteoblast differentiation. It (1 μM) combined with MK-2206 (2 μM) completely reverses the resistance of RSK-expressing MCF7 cells. |
| In vivo |
Binimetinib (MEK162) demonstrates dose-related inhibition of ankle swelling in rat adjuvant-induced arthritis (AIA) models, significant at 10 mg/kg and 30 mg/kg when compared to vehicle control. This compound also shows dose-related inhibition of serum IL-6 concentration in rat adjuvant-induced arthritis (AIA) models, with complete inhibition at 10 mg/kg when compared to vehicle control. At 30 mg/kg, it demonstrates dose-related inhibition of relative spleen weights in rat adjuvant-induced arthritis (AIA) models. Additionally, it significantly inhibits bone resorption and inflammation with delayed dosing when compared to vehicle in rat adjuvant-induced arthritis (AIA) models. ARRY-438162 (10 mg/kg, po, bid) reduces disease severity in a dose-related manner in rat collagen-induced arthritis (CIA) and rat adjuvant-induced arthritis (AIA) models. It (po, bid) inhibits increases in ankle diameter by 27% and 50% at 1 mg/kg and 3 mg/kg in the rat collagen-induced arthritis (CIA) model, while ibuprofen has 46% inhibition. ARRY-438162 (10 mg/kg, po, bid) significantly inhibits lesions (inflammation, cartilage damage, pannus formation and bone resorption) by 32% and 60% at 1 mg/kg and 3 mg/kg in the rat collagen-induced arthritis (CIA) model. It inhibits AIA ankle diameter 11% and 34% at 3 mg/kg and 10 mg/kg in rat adjuvant-induced arthritis (AIA) models. When combined with BEZ235, it (6 mg/kg, BID) results in a significant reduction of tumor growth in immunodeficient mice injected with MCF7 cells. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | MEK / p-MEK / ERK / p-ERK p-KIT / KIT / ETV1 |
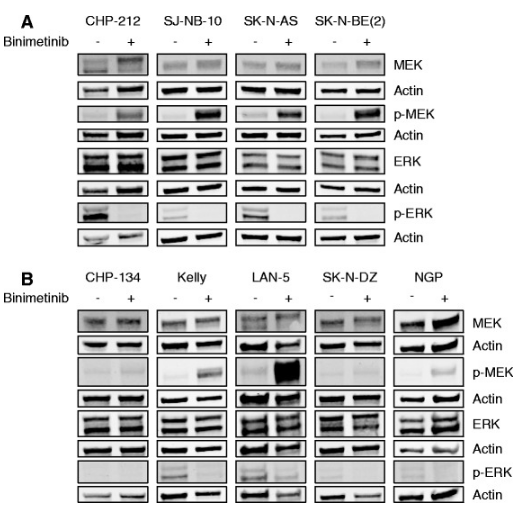
|
26925841 |
| Growth inhibition assay | Cell viability |
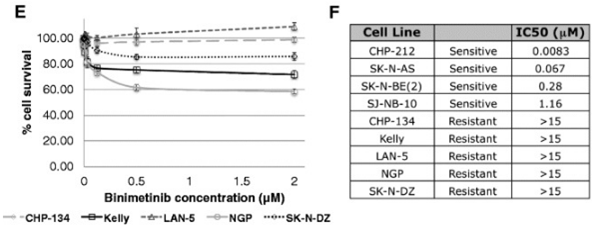
|
26925841 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06207656 | Not yet recruiting | Colorectal Cancer |
Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD)|Merck S.L. Spain|Pierre Fabre Ibérica S.A. |
January 19 2024 | Phase 2 |
| NCT05286788 | Recruiting | Adamantinous Craniopharyngioma|Recurrent Adamantinomatous Craniopharyngioma |
Nationwide Children''s Hospital|Children''s Hospital Colorado |
April 10 2023 | Phase 2 |
| NCT05810740 | Completed | Melanoma|BRAF V600 Mutation|Unresectable Melanoma|Metastatic Melanoma |
Pierre Fabre Medicament|Biotrial |
August 31 2022 | Phase 1 |
| NCT05195632 | Active not recruiting | Non-Small Cell Lung Cancer |
Pierre Fabre Medicament |
June 2 2022 | Phase 2 |
| NCT05767879 | Recruiting | Melanoma Stage III|In-Transit Metastasis of Cutaneous Melanoma |
Leiden University Medical Center|Pierre Fabre Laboratories |
January 1 2022 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
Could you please clarify whether the formulation in vivo for S7007 is clear or not?
Answer:
It can be dissolved in 5% DMSO+45% PEG 300+ddH2O at 5 mg/ml clearly for injection.






































